Complete Process to Apply for Wholesale Drug License in India
Japsanjam Kaur Wadhera | Updated: Jan 23, 2021 | Category: Drug
To start the business of manufacturing and selling drugs, it is important for a business to obtain drug license in India. The person who is willing to undertake a wholesale drug business must obtain a license from the Food Safety and Drug Administration. Form 20B, 20G can be filed by the applicant to obtain wholesale drug license. It is granted by the respective State Licensing Authority. The business of manufacturing and selling of drugs is governed by the provisions of Drugs and Cosmetics Act, 1940[1]. This article will discuss about the complete process to apply for wholesale drug license in India.
Table of Contents
Need for the Wholesale Drug License in India
A wholesale drug license is required to distribute, sell and store the medicines. It can be issued from the appropriate government authority. The license is helpful to follow all the rules and regulations laid down by the regulatory authority.
Conditions to obtain Wholesale Drug License in India
In order to obtain wholesale drug license in India the below mentioned conditions are required to be fulfilled: –
- The business premises should be a minimum of 15 square meters.
- The business premises must have air conditioner and refrigerator on the premises.
- The application for the wholesale drug license can be made under the personal supervision of the registered pharmacist or the competent person. In certain states a wholesale drug license is provided to only those entities or companies who have persons with a diploma or degree in pharmacy from a reputed and recognized university or institution.
- The competent person or the pharmacist must be a graduate with at least 1 year experience in dealing with drugs or be SSLC passed have 4 years experience in dealing with drugs.
- The drug license, after it has been obtained must be displayed clearly on the premises for the purpose of any inspection in future.
- The business premised must not be an open-roofed area that is, it must be closed and well-sanitized area.
- The applicant is required to get mandatory GST registration number.
How to Apply for Wholesale Drug License in India?
In order to apply for the wholesale drug license in India, the below mentioned steps are required to be followed:
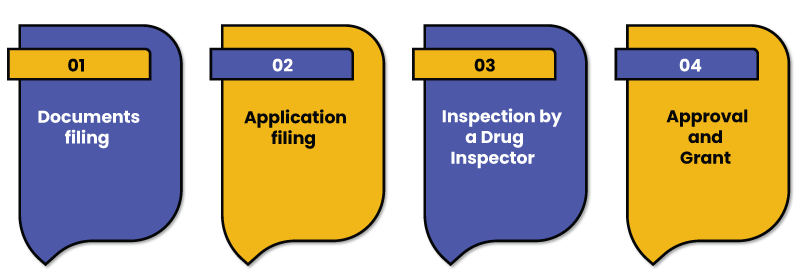
Documents filing
The applicant must ensure that he has all the required documents that are needed to be submitted and must ensure that he meets the prescribed requirements and conditions.
Application filing
The applicant shall file the application for drug license online. While filing the application, the applicant is required to upload all the scanned copies of the required documents. The application shall be accepted once the payment of the required fees has been done.
Inspection by a Drug Inspector
Once the application is filed, the documents and the application shall be reviewed by the drug inspector in person. The drug inspector shall visit the store and ensure that the business meets all the prescribed requirement. Post the verification, an approval is given by the drug inspector to proceed further.
Approval and Grant
The final step in obtaining wholesale drug license in India is when the documents are verified and all the requirements are met, the application shall be approved by the Drug Licensing Authority and the license is granted. However, the license is required to be renewed.
Documents required to apply for wholesale drug license in India
The documents required to apply for wholesale drug license in India are generally:
- Two applications filed through Form 19.
- Challan fees deposited for the license.
- Declaration Form.
- Cover letter stating the purpose of the application.
- Receipt of property tax.
- Rent agreement if the premise is rented.
- Ownership documents if the premise is self owned.
- Memorandum of Association (MOA) and Articles of Association (AOA), Incorporation Certificate (if the business entity is an LLP or a Company).
- If the entity is a partnership then a partnership deed.
- If the entity is a private limited company then the list of directors.
- Board resolution.
- Affidavit of the Pharmacist.
- Affidavit of the Proprietor.
- Qualification certificate of the pharmacist or the competent person.
- Identity proof of the proprietor.
- Appointment letter.
- Proof of the availability of the storage space for items that are required to be stored in low temperature such as, refrigerator, air conditioners etc.
- Photograph of the pharmacist/ proprietor/ competent person.
Eligibility to apply for wholesale drug license
The following persons/entities are eligible to apply for wholesale drug license: –
- Any company/ LLP/ partnership firm/ person can apply for the drug license.
- A competent person (registered pharmacist from the state pharmacy council) with experience can apply for the wholesale drug license.
Validity of the license
The wholesale drug license is valid for a period of 3 years. The license should be displayed clearly at the place of the business during the business hours.
Conclusion
Drugs and medicines are a necessity and essential products. Understanding its importance the government has laid down a strict application process for obtain a drug license. Starting a wholesale drug business in India is not very complex but yet a person needs to prepare for it. All the formalities are required to be completed in the process of issuing drug license. This ensures that the drug wholesalers comply with all the provisions. To apply for wholesale drug license in India the applicant is required to apply in accordance with the rules and regulation of the Drugs and Cosmetics Act, 1940. The provisions ensure that the drug business is undertaken and performed by a qualified and competent person.
Since the safety of the people is the most important priority, the government ensures that the drugs do not risk the lives of the people. The Central Drug Control Organization (CDSCO) is the regulatory body for pharmaceuticals and medical devices. CDSCO is responsible for the laying down the standards for drugs and coordinate with the activities of the State Drug Control Organization (SDCO) by providing expert advice to bring about uniformity in the enforcement of the provisions of the Drugs and Cosmetics Act.
Also, Read: How to get a Drug Distribution License in India?