An Overview of Drug License
A Drug License is also known as Pharmaceutical License, and this License is required for all businesses form involved in the distribution of medicines & pharmaceutical supplements. According to Section 3(b) of the Drugs & Cosmetics Act, 1940, drugs comprise all devices & medication for animals as well as human beings internally or externally, and all substances proposed to be used for or in the mitigation, treatment, diagnosis, or prevention of any disorder or disease in human beings or animals. The definition was amended in 1964 to comprise Ayurvedic & Unani Drugs. Drug License is mandatory to do business in the area of Drugs & Cosmetics in India. The provision for this has been made in the Drugs & Cosmetics Act, 1940, which is relevant to the whole of India.
If in case a business has an operating unit in two or more states, it has to obtain a License for each state in which the business is being carried on. Drug License Registration is location-specific. It means if the drugs are stocked or sold for sale and distribution at more than one place, then the application shall be made to every state, and a license shall be issued regarding each such place except for migrant vendors.
Purpose of Drug License
Access to medicines & drugs must be restricted & regulated to make sure that such goods or products are not misused or abused by individuals. Hence, all wholesalers, manufacturers, dealers, pharmacists, retailers, and importers of cosmetics, drugs, ayurvedic, Siddha & Unani drugs have to compulsorily obtain Drug License under the Drugs & Cosmetics Act, 1940.
The purpose of this License is to grant permission to allow enterprises or individuals to engage in businesses regarding Drugs & Cosmetics. No individual or enterprise can run a business dealing drugs, cosmetics, or medicines without getting a Drug License in India.
The Drugs & Cosmetics Act, 1940 and Rules, 1945 help the Government regulate & monitor the quality of drugs sold in India. The Government practices control over drugs from the raw material stage during the sale, manufacture distribution and tills it is sold to a patient or customer by a pharmacist in a hospital, retail pharmacy or dispensary. Also, the Government exercises control concerning the import & export of medicines, sale of the drug to a minor and the consumption of Schedule H&X Drugs, etc., which requires monitoring & cautious execution.
Different Types of Drug Licenses in India
Following are the different types of licenses issued depending upon the type of business for which the License is obtained:
- Manufacturing Drug License
All the manufacturers dealing with ayurvedic, allopathic, cosmetics, or any other medications or drugs, as mentioned under the Drugs and Cosmetics Act, 1940, are required to obtain Manufacturing Drug License. This is the state license that is granted by the respective state government where the unit is located.
- Loan Drug License
The Drug Manufacturer who do not possess any land on his own but is willing to manufacture drugs by using his brand name on the land to which the License is already granted.
- Import Drug License
Any dealer who is importing the products for manufacturing drugs or is involved in the business of importing drugs in India shall mandatorily obtain this specific License.
- Multi-Drug License
Multi-Drug License is obtained by entities operating in more than one state or having multiple units.
- Sale Drug License: A sale License is issued for the sale of drugs and it has the following bifurcations:
- Wholesale Drug License: Wholesalers who are engaged in the business of pharmaceuticals shall mandatorily acquire a wholesale drug license from the CDSCO (Central Drugs Standard Control Organization).
- Retail Drug License: This License is acquired by the retailers engaged in either in pharmaceuticals business, or stand-alone pharmacists, etc. The same is acquired by applying to the State Pharmacy Council.
Who issues Drug License in India?
Drug license acts as an authority given by the Government of India to the owner of the drugstore or pharmacy. The Drugs and the Cosmetics Act, 1940, is the governing force for the issuance of this License in India. This act provides the provisions for carrying out the business of medicines, drugs, or cosmetics in India.
In India, the License is granted by the following listed authorities:
- State Drugs Standard Control Organization (SDSCO): As per the provisions of the Drugs and Cosmetics Act, 1940, the sales, distribution, and manufacture of drugs are regulated by the state authorities.
- Central Drugs Standard Control Organization (CDSCO): According to the Drugs and Cosmetics Act of 1940, the CDSCO is responsible for approving the newly made drugs and clinical trials of the said drugs. It also controls the quality of the drugs imported and coordinates with the SDSCO.
Key Requirements for obtaining Drug License Registration in India
The following are the essential key factors to be taken into consideration while applying for the License:
- Premise Area
A minimum area of 10 sq meters is needed for setting up a retail pharmacy or a medical shop. However, in the case of Retail cum Wholesale Drug business, the required minimum area shall increase to 15 sq meters.
- Proficient Individual or Pharmacist:
The pharmacist or proficient individual should be qualified in the case of a retail business. In the case of a wholesale business, the individual should be a graduate with at least one year of experience or an undergraduate with four years of experience.
- Storage Facility
Proper storage facilities must be ensured by implementing the refrigerator, cold storage facility, or other similar places for the storage of drugs and medicines as they shall be restored in cool places.
- Technical and Expert Staff
A Retail pharmacy must have an experienced and technical staff with in-depth knowledge of the same. A qualified pharmacist is a must for obtaining a License. However, in the case of Wholesale Drug License, the said staff must either be a graduate with a minimum of one-year of experience or an undergraduate having four years of experience.
- Inspection by the Drug Inspector
Before the issuance of a License, the Drug Inspector who has jurisdiction of the area will visit the premises for which the Drug License has been applied and will verify all the particulars furnished with the application. If in case needed, he may also take the premise's measurement and may also interview the competent person.
What are the Different Forms of Drug License?
The different forms needed for obtaining various types of Drug License are mentioned below:
|
Form Number |
Purpose of the Form |
|
Form 19 |
This form is used for the grant of renewal of a License to sell, stock, distribute, or exhibit drugs apart from those specified in Schedule X. |
|
Form 19A |
Application for renewing or granting a restricted license to stock, sell, stock, or offer for sale, or distribute drugs by retail through dealers who don't engage the services of an eligible individual or person. |
|
Form 19B |
This form is used to sell, stock, offer for service or exhibit, or distribute Homoeopathic Medicines. |
|
Form 19C |
Application for renewal/grant of a license to stock, sell, exhibit/offer for sale, or distribute drugs specified in Schedule X. |
|
Form 20 |
Application for License to stock, sell, or exhibit or offer for sale or distribute drugs by retails other than those prescribed under Schedules C, C(1), and X. |
|
Form 20A |
Application for restricted License to stock, sell, or exhibit or offer for distribution or sale drugs by retailers other than those prescribed under Schedules C, C(1), and X for dealers who don't engage the services of registered pharmacists. |
|
Form 20B |
Application for License to stock, sell, or exhibit or offer for sale/distribute byWholesale other than those prescribed under Schedules C, C(1), and X. |
|
Form 20C |
Application for License to stock, sell, or exhibit or offer for sale/distribute Homoeopathic medicines by retails. |
|
Form 20D |
Application for License to stock, sell, or exhibit or offer for sale/distribute Homoeopathic by wholesale. |
|
Form 20E |
Application for certificate of renewal of License to stock, sell, or exhibit or offer for sale or distribute Homeopathic medicines. |
|
Form 20F |
Application for License to stock, sell, or exhibit or offer for sale/distribute by retail drugs specified in Schedule X. |
|
Form 20G |
Application for License to stock, sell, or exhibit or offer for sale/distribute by wholesale drugs specified under Schedule X. |
|
Form 21C |
Application for the renewal of License to stock, sell, or exhibit or offer for sale or distribute drugs. |
|
Form 24 |
This form is for either the renewal of a license or for the grant of a license/to manufacture for sale or for the distribution of drugs other than those which are described in Schedule C, C(1) and X. |
|
Form 24A |
This application for either the grant of a loan license/renewal of a loan license to manufacture for sale/distribution of drugs other than those specified in Schedule C, C (1) and X. |
|
Form 24B |
This form is for grant or renewal of a license to repack for sale/distribution of drugs, being drugs other than those described in Schedule C and C(1), excluding those specified in Schedule X. |
|
Form 24C |
This is for the renewal or grant of a license to manufacture for sale or for distribution of Homoeopathic medicines or a license to manufacture potentised preparations from back potencies by license owners holding a license in Form 20-C. |
|
Form 24D |
Application for the grant/renewal of a license to manufacture for sale of Ayurvedic or Siddha or Unani Drugs. |
|
Form 24E |
Application for the renewal or grant of a Loan License to manufacture for sale of Ayurvedic (including Siddha) or Unani Drugs. |
|
Form 24F |
This form is for the grant/renewal of a license to manufacture for sale/distribution of drugs specified in Schedule X and not specified in Schedule C and C(1). ' |
|
Form 27 |
This is for renewal or grant of a license to manufacture for sale or for distribution of drugs specified in Schedule C and C(1) excluding those specified in Schedule X and Part XB.’ |
List of Vital Documents Required for Obtaining a Drug License in India
Following are the vital documents needed to obtain a Drug License in India:
- Identity proof of director or partner or proprietor;
- Key plan & site plan of the premises;
- Constitution of the Entity – MOA (Memorandum of Association), AOA (Articles of Association) for a company. Partnership Deed, LLP Agreement in the case of LLP) and Partnership;
- Copy of Board Resolution allowing obtaining of a license;
- In the case of a rented premise, a copy of ownership documents of property or rental agreement & a NOC (No Objection Certificate) from the owner;
- Proof of availability of storage space such as cold storage, refrigerator, etc.;
- Challan as proof of depositing fee;
- Affidavit concerning non-conviction of Partner or Director or Proprietor and the firm;
- The affidavit from the registered pharmacist or competent person;
- Cover letter with name & designation of the applicant;
- Declaration form in a prescribed format;
- Qualification certificate of the applicant;
- For a pharmacist at a retail sale:
- Qualification proof;
- Appointment letter;
- Registration of Local Pharmacy Council
- For a Pharmacist at a Wholesale Sale:
- Experience Certificate
- Qualification Proof
- Appointment Letter
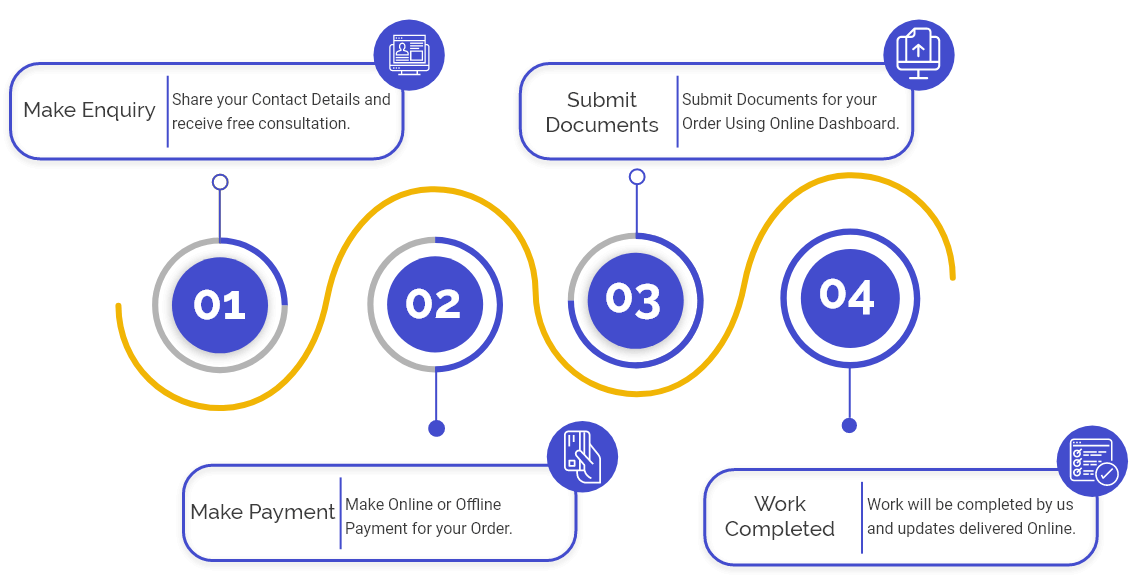
Procedure for Obtaining Drug License in India
The steps involved in obtaining the Drug License in India are listed below:
-
Visit the Respective Drug Controlling Authority Portal: First, the applicant needs to visit the respective authority (CDSCO, SDSC), or Ayush) depending on the type of License he or she is applying for since each Drug Controlling Authority is accountable for the issue of different types of Drug licenses.
-
Filling of Application: Then, the applicant must file the application for the License on the respective authority website and the applicant must fill out all the details asked in the application form.
- Upload Documents: After filing the application form, the next step is to upload the documents & submit the form along with the applicable fees. Also, the applicant must keep all the documents updated before filing the Drug application.
- Visit by the Drug Inspector: After receiving the application, the Durg Inspector will visit the company premises, drug store, or shop for the verification of documents & correctness of facts mentioned in the application.
- Issuance of License: After the completion of the inspection of the premise and verification of all the documents and applications. The Drug Controller will issue the License.
What is the difference between a Drugstore and Pharmacy?
|
Drugstore |
Pharmacy or Dispensary |
|
A Retail store where both the prescriptive and non-prescriptive drugs are sold together with other first aid and similar items. |
A place where only prescriptive drugs are dispensed or provided |
|
These drugstores earn a majority share of their revenue by way of the products, which include both health and beauty products. |
In this case, prescribed medicines act as the only source of income for individuals. |
Frequently Asked Questions
The term “Drug License” denotes the permission granted by the government to deal with drugs and cosmetics. It is a must requirement for an individual who wants to engage in or want to do business in Wholesale, Manufacturing, Retail, and Distribution of Pharmaceuticals.
The authority to grant Drug License to the applicant is only with the State Government.
If an applicant wants to obtain a Drug License for his/her Medical Shop or a Chemist Shop, then he/she must have a minimum area of 10 sq. meters, Air Conditioners, and Refrigerators installed at the Store Medicines. Moreover, the shop owner must hire qualified and professional personnel as staff at the shop.
For the Renewal of Drug License, the applicant needs to fill out an application form and submit all the required documents along with the form. Further, after filing the application form, the applicant requires to submit the same to the Drug Control Department, along with the prescribed fees. After that, the authorities will review the application form submitted, and if satisfied, will issue a new certificate of Drug License to the applicant.
No, a pharmacist cannot open and run a clinic to diagnose disease and recommend medicines. The same had been notified and declared by the Pharmacy council of India. Also, there are no provisions prescribed in the Pharmacy Practice Regulation for a pharmacist to practice pharmacy.
The term “Licensed Drug” denotes a drug that undergoes a strictly regulated process. It includes both lab researches and trial tests. Once the drug had successfully completed all the tests and trials, it will be issued a license.
A License required for manufacturing drugs as per the Drugs and Cosmetics Act, 1940, is known as Drug Manufacturing License. Further, the applicant can apply online for obtaining Drug Manufacturing License from the State Drugs Controller cum Licensing Authority.
To obtain a Drug Distribution Licence, the applicant needs to file an application 19 and the prescribed documents such as the Identity Proof, Ownership Documents of the Premises, Affidavit of Registered Pharmacist, etc. with the authorities.
In India, opening an Independent Pharmacy needs a high-level investment. The term “Investment” includes the form of furniture and fitting, legal paperwork, and any other investment that will cost an individual around Rs 60000 approx.
The drugs that are classified and bifurcated as per the Drugs and Cosmetic Rules, 1945, are known as the Schedule C Drugs. Further, various biological products are included in the category of Schedule C Drugs. It also includes Toxins, Sera, Antigen, Antitoxins, etc.
The term “Form 21C” means a license to stock, sell, exhibit, or offer for sale or distribute retail drugs mentioned in Schedule C and C1. Moreover, a manufacturer who wants to sell, stock, etc. also need this license.
The applicant who wants to obtain a Retail Drug License must have an adequate area of not less than 10 sq. metres. Further, the area should be equipped with all the proper storage facilities for preserving and conserving the properties of drugs. Lastly, the applicant (Competent Person) must mandatorily be registered as a Registered Pharmacist with the Pharmacy Council.
Essentials for obtaining a Wholesale Drug License is the same as the Retail Drug License. However, in this case, the applicant must have passed Matriculation or is has equivalent four years of experience in dealing with the Drugs or is holding a degree, together with one of year experience in dealing with Drugs.
The applicant’s Chemist or Medical shop must have an adequate area of not less than 15sqr.mtrs.
A Written complaint about the questionable quality of Drugs can be filed at the Drugs Control Department, Govt. of N.C.T. of Delhi, at F-17, Karkardooma, Shahdara, Delhi-32. Further, the complaint made must be indicating the Name and Address of the complainant, Name, and other particulars of the concerned drug and the nature of the complaint.
Several provisions dealing with the Drug license are provided under the Drugs and Cosmetics Act, 1940, and the rules thereunder. Moreover, the information and details can also be obtained in person from the drug inspectors placed in the Drugs Control Department.
The basic features of a Drug License include obtaining Drug License for drugs, medicine, or cosmetics business; the Drug License shall be issued for a commercial premise; Drugs and Cosmetics business needs to mandatorily comply with the requirement and conditions prescribed by the law and issuing authorities; Lastly, the Holder of Drug License must display the license issued all the time in the place of business.
Yes, an individual needs to obtain different a Drug License for each unit operating in any part of India. For example: For example, if a drug business has its unit operating in two separate states, it shall obtain the drug License for both the operational unit.
The books of accounts, forms, registers, and other related documents shall be maintained in such a way as may be prescribed by the licensing authority at the time of issuance of such license. Moreover, it is the duty of the license holder to intimate about every change and modification that happened in his business.
Form 20B and Form 21B are required by the applicant got for obtaining the wholesale Drug License for the sale and redistribution of Allopathic Drugs under the Drugs and Cosmetics Rules.
A Drug License for the sale of drugs can only be granted for those premises that are commercial premises or any other premise independent of residence. Before starting a business of drugs, it is always advisable to take guidance from the guidelines issued by the state drug office for this purpose.
Yes, obtaining Drug License is mandatory before starting any business dealing in drugs, whether it is Ayurvedic Medicine, Allopathic Drugs, Homeopathy or Unani, to manufacture, distribute or sell the drugs.
If in case a business dealing in drugs is operating in more than two states, then there is a need for the owner to obtain a drug license in every state in which the said business is being carried on. Further, the Drug license is a location-specific license, and hence within a state application, all sites must be included.
The Drugs and Cosmetics Act, 1940, acts as the governing and regulating force for the business dealing with drugs.
In India, the Drugs and Cosmetic Act, 1940, aims at ensuring the effectiveness, safety, and conformity of the drugs and cosmetics sold to the state quality standards. Moreover, the drugs or cosmetics mentioned under schedule X are eligible to be imported only after obtaining Drug License.
The main two advantages of a Drug License include freedom from the fear of being caught from selling drugs without acquiring a Drug License. Secondly, it assists in the expansion and growth of the wholesale drug selling business, as retailers would never hesitate in dealing with a person who has obtained the license from the concerned authority.
Licenses in the Form 20-A and 21-A are needed for the sale of Restricted Drugs, commonly known as Household Remedies or Medicines. Further, for the sale of such drugs, services of a registered pharmacist and prescription of a Doctor (RMP) are not needed.
According to Rule 33 of the Drugs and Cosmetics Acts, 1940, and Rules, Form 11 is granted for the purpose of allowing import of the small quantities of drugs that are used for examination testing or analysis.