Cancelling of Drug Patents: A Boon or a Ban?

Karan Singh | Updated: Jun 11, 2021 | Category: Patent
In India, Patents rights were commenced for the first time in the year 1856 & 1970. The Patents Act was passed that cancelled all earlier legislations available in India. The Patents Act provides that any invention which fulfils the criteria of originality, usefulness and non-obviousness can be the subject matter for Patent Registration. India already has a product Patent system for all the creations or inventions under the Patents & Designs Act. The Government announced the new Patents Act in the year 1970 that barred pharmaceuticals and agrochemical goods from eligibility for Patent Registration. This abolition was started to break away the reliance of India on drug imports in bulks and formulations & also deliver for the independent pharmaceutical industry development. In this write-up, we will disucss the cancelling Drug Patents.
Table of Contents
What is the Impact of WTO on Drug Patents?
The establishment of WTO (World Trade Organisation) has led to extraordinary growth in world trade. The TRPS Agreement was discussed at the time of the Uruguay round that concern about the GATT (General Agreement on Tariffs and Trade) and of the main reasons for combined Intellectual Property issue in the structure of GATT was that of the drugs industry. On 15th April 1994, India signed the GATT Agreement, thus making it mandatory to fulfil the GATT terms comprising the TRIPS Agreement.
Hence, India is required to fulfil the minimum standards mentioned under the TRIPS Agreement concerning the Drug Patent. The Patent Law of India must now include provisions concerning the Patents availability of Patents for both pharmaceutical inventions & drugs. Patent Registration is to be permitted for twenty years to any invention of pharmaceutical drugs or invention process of that specific drug that satisfies the confirmed criteria.
An Overview of Drug Patents
Pharmacy entities spend lots of money on testing and developing drugs. So to aid them to recover the money spent on the R&D, the Government permits them exclusive right for their Intellectual Property (IP). Drug Patents permits pharmacy entities to recover their investment in inventing a specific drug, invest in innovation, & push them to develop more reasonable drugs.
Such Drug Patents deliver the pharmacy entities with protection, providing them with exclusive marketing rights & sell the drugs. Once the license is cancelled, other entities can use the published records concerning the drug manufacturing process & can develop an inexpensive version of the drug, also known as generic medicines.
All over the globe, the validity of Drug Patents is for twenty years only. In Indian, such Patents are also valid for twenty years, after which the common drug manufacturer can sell their drug version. Such Patents expiration gives the common companies the green signal to start selling and marketing, thus ending the control.
Benefits of Expiring of Drug Patents
Expiring of such Patents helps in finishing the control over any drug. Once the general medicines hit the market, the drugs price falls instantly, making the drugs cheaper to the generic public. This can also increase and promote market competition, which leads to more variety and option for the generic public. It also makes sure that essential and life-saving drugs reach the public at an affordable price. Besides, pharmacy entities buy the rights to patents of drug for sale from other business and spread their control right over that drug.
Is it Fair to broaden the patents of Drug?
The expiration of such Patents in India brings high price relief to the public as competition from the available versions makes cost low. As an outcome, most professional feel that widens such Patents put the public at a drawback. Hence, the Government must come out with severe laws that prevent pharmaceutical entities from widening the patents of drug.
Cancelling of Drug Patents
Protection of Patent ensures that the innovation furnishes the pharmacy companies with a commercial temptation. Huge pharmacy entities feel no wish to develop & experiment with new drugs if no commercial incentives are involved. The Paten for drugs encourages Research & Development and also protects the researcher’s interests.
Whereas the Patent system may not be ideal but doing away with it could lead to a pause in research. If we cancel patents for drugs, no one will try to develop new drugs in fear of getting demoralised. Therefore, the Patent system has its troubles; the best solution would be to restructure and not cancel such Patents.
Government should also focus on a considerate balance between originality and affordable pricing. Though it is necessary, we deliver drug manufacturers a profit purpose, but it shouldn’t come at the price of bankrupting clients. Laws should be put in place to prevent corporations from not spending on research & just buying patents of drug for sale. The Patent system must be moved forward for innovation and not support profit maximisation.
What is the Impact of the Patent Law of India on the Pharmaceutical Company?
Following is the impact of the Patent Law of India on the Pharmaceutical Company:
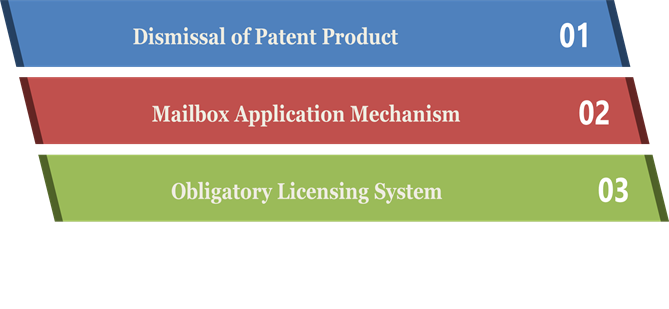
- Dismissal of Patent Product: The report on the Indian Patent Law revision that the Patent Law Commission[1] submitted in the year 1959 that Justice N. Rajagopala Ayyangar led tells that in 1959 foreigners held 80% – 90% of the Patents of India. Out of which 90% of the products were not produced in the Indian Territory.
- Mailbox Application Mechanism: This mechanism was available at the time of the transition period, which permitted India to meet the TRIPS requirements and also to follow with the development need of India. The transition period of India to satisfy the requirements of TRIPS concerning pharmaceuticals was for a time of ten years begins from 1995.
- Obligatory Licensing System: This system creates more chances of voluntary licensing conciliation between the Indian Pharmaceutical entities & multi-national company. As per the Patent Act of India, after the pause of three years from the date of Patent Registration, any individual interested can create an application to the Patent Controller. The candidate is required to obtain a voluntary license from the Patent owner before applying for a compulsory license. It must be done within six months from the main request; the candidate is allowed to file for a mandatory license.
Conclusion
Cancelling of Patents for drug in India aids in concluding the control of substantial pharma entities over any drug. Hence, as mentioned above, cancelling such Patents is suitable for the public as the costs of the drugs decrease. The Indian Government should desire to maintain a balance between novelty & rational costing. It is vital to furnish drug manufacturers with a profit objective but not the price of bankrupting the clients. Laws must be provided to prevent entities from not spending on research & just buying patents of drug for sale.
Read our article:An Overview of Biotechnology Patents in India