Restrictions on Manufacture, Sale and Distribution of Drugs

Shivani Jain | Updated: Jul 17, 2020 | Category: Drug
In today’s era, drugs have become an inevitable part of everyone’s lives. We need them to preserve or restore our health. Hence, they are also known as life saving remedies. Further, in India, the Drugs and Cosmetics Act of 1940 acts as governing law for drugs and cosmetics. Furthermore, in this blog, we will discuss the laws relating to Restrictions on Manufacture, Sale and Distribution of Drugs.
There were two main aims behind the enforcement of Drug laws in India[1] . The first was to provide drugs and cosmetics to the consumers at a fair price. And second to curb the exploitation of consumers from adulterated, misbranded drugs and cosmetics.
Table of Contents
Concept of Standard Quality Cosmetics and Drugs
The cosmetics and drugs which are made as per the standards specified in the second schedule are known as standard quality cosmetics and drugs.
Further, the Central Government, together with the Drugs Technical Advisory Board[2] , can amend the second schedule.
Furthermore, the government needs to give at least three months notice before amending the schedule second.
Restrictions on Manufacture, Sale and Distribution of Drugs and Cosmetics
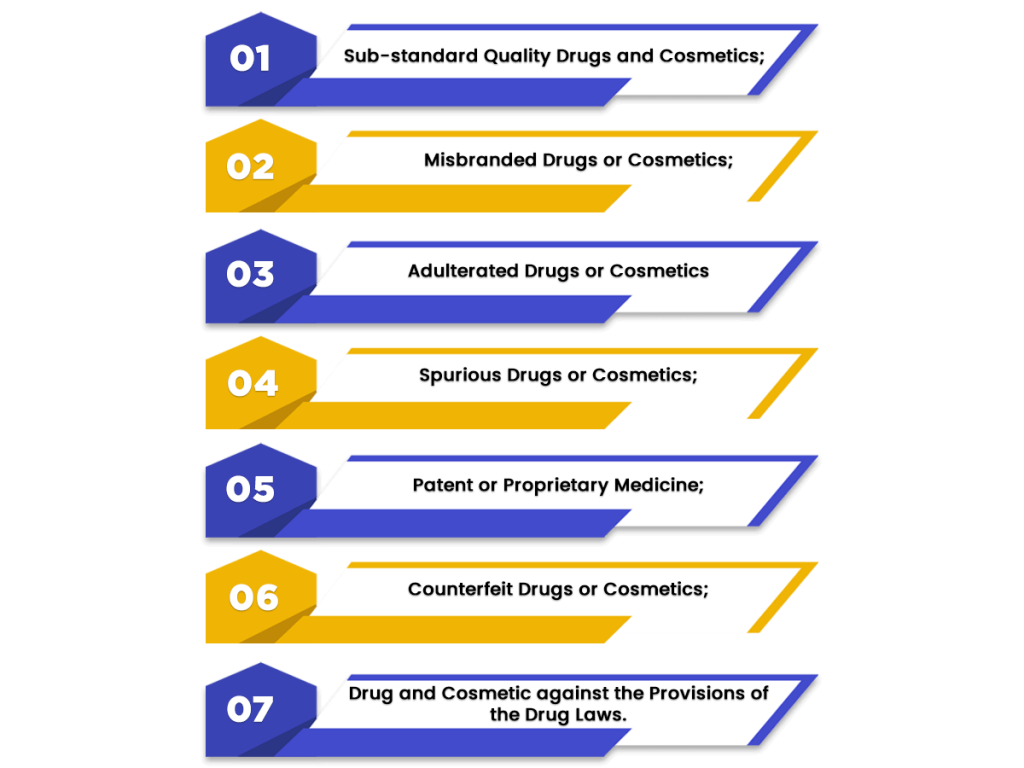
In India, the drugs and cosmetics that have been restricted from manufacture, sale, and distribution are as follows:
- Sub-standard Quality Drugs and Cosmetics;
- Misbranded Drugs or Cosmetics;
- Adulterated Drugs or Cosmetics;
- Spurious Drugs or Cosmetics;
- Patent or Proprietary Medicine;
- Counterfeit Drugs or Cosmetics;
- Manufacture, Sale and Distribution of drugs and cosmetics against the provisions of this act.
Concept of Sub-standard Quality Drugs and Cosmetics
Any pharmaceutical product or cosmetic that does not qualify the prescribed quality standards and specifications is known as Sub-standard Quality Drugs or Cosmetics.
Further, these specifications and standards are regularly assessed, reviewed, and approved by the NMRA before the drug is authorised for marketing. Furthermore, the acronym NMRA stands for the National Medical Regulatory Authority of India.
Concept of Misbranded Drugs or Cosmetics
The definition of Misbranded Drugs is given under section 17 of the Drugs and Cosmetics Act, 1940. As per this definition, a Drug will be considered as Misbranded in the following situations:
- If it is coated, colored, polished or powdered to conceal the damage or make it appear as of better quality than it really is;
- If it is not properly labeled;
- If the container or the label of the drug bears any design, statement, or logo that makes any false or misleading claims.
Further, the definition of Misbranded Cosmetics is given under Section 17C of the Act. As per this definition, a Cosmetic will be considered as Misbranded in the situation as follows:
- If the product contains unspecified color;
- If it is not labeled properly;
- If the label or container of the product bears any logo or design, which makes any misleading or false claims.
Concept of Adulterated Drugs or Cosmetics
The definition of Adulterated Drugs is given under section 17 A of the Drugs and Cosmetics Act, 1940. As per this definition, a Drug will be considered as Adulterated in the situations as follows:
- If the product contains any putrid, filthy or decomposed substance, whether in whole or in part;
- If the drug has been packaged, prepared, or stored under insanitary or unhygienic conditions, whereby it maybe got contaminated with filth or have been rendered injurious and harmful to health;
- If the container of the product is made of any deleterious or poisonous substance which may render the components injurious to health;
- If for color, the drug contains any color other than the one prescribed;
- If the product contains any toxic or harmful ingredient which may render it injurious to health;
- If the product includes any substance, which thereby reduces its strength or quality.
Further, the definition of Adulterated Cosmetic is given under section 17 E of the Act. According to the definition, a drug will be considered as Adulterated in the following situations:
- If the product contains any putrid, filthy or decomposed component;
- If the product has been packed, prepared or stored under unhygienic conditions;
- If the cosmetic comprises of any poisonous or deleterious substance;
- If the product contains any color other than the prescribed one;
- If the item includes any toxic or harmful component, which is injurious to health;
- If the product contains any substance which thereby reduces its strength or quality.
Concept of Spurious Drugs or Cosmetics
The definition of spurious drugs or cosmetics is given under sections 17 B and 17 D of the Act, respectively. The situations in which the manufacture, sale and distribution of drugs or cosmetics become spurious are as follows:
- If a drug or cosmetic is prepared under a name which belongs to another cosmetic or drug;
- If the product is imitation or substitute of an already existing cosmetic or drug;
- The label or the container of the product bears a fictitious name. The term “fictitious” denotes company name which does not exist;
- If the product has been substituted by another drug or cosmetic;
- If it claims to be the product of a manufacturer of whom it is not really a product.
Concept of Patent or Proprietary Medicine
The term “Patent or Proprietary Medicine” means a drug, which is a prescription or remedies for human beings. However, the said drug has not received recognition from the WHO or the authorities working on behalf of the Central Government.
Therefore, the manufacture, sale and distribution of drugs of such type have been restricted by the government.
Concept of Counterfeit Drugs or Cosmetics
The WHO (World Health Organisation) defines “Counterfeit Drugs or Cosmetics” as the product which has been fraudulently and dishonestly mislabeled regarding its identity or history of the source. Further, it clarifies that both branded and generic products are included in Counterfeit Drugs or Medicines.
Furthermore, this definition also includes the following listed:
- Product with correct ingredients;
- Product with wrong ingredients;
- Drugs and Cosmetics without active ingredients;
- Drugs and Cosmetics with too many active or insufficient ingredients.
Prohibition on the Manufacture, Sale and Distribution of Drugs and Cosmetics
As per section 18 of the Act, the situations relating to the prohibition on the manufacture, sale and distribution of Drugs and Cosmetics are as follows:
- When the drug is of Sub-standard Quality, Misbranded, Spurious or Adulterated;
- When the cosmetic is of Sub-standard Quality, Misbranded, Spurious or Adulterated;
- If the drug belongs to the category of Patent or Proprietary Medicine;
- If a drug purports or claims to prevent, cure or mitigate, any disease or ailment;
- When a drug or cosmetic is manufactured in contravention to the stipulations prescribed;
- When a cosmetic comprises of harmful and unsafe ingredient;
- Sale and Distribution of any product manufactured in contravention of the Act;
- Stock or exhibit any drug or cosmetic which has been prepared or imported in violation of the Act.
Exceptions to Section 18 of the Drugs and Cosmetics Act
The exceptions to section 18 of the Drugs Act are as follows:
- The provisions of this section do not apply to the manufacture of drugs in small quantities. However, such manufacturing must relate to analysis, test or examination of the drug, subject to the prescribed conditions;
- The Central Government, together with the Drugs Technical Advisory Board, can allow the manufacture, sale and distribution of drugs or cosmetics of sub-standard quality.
Disclosure of the Premises
As per section 24 of the Act, every manufacturer, seller, or distributor of drugs or cosmetics need to make a complete disclosure of the place or premise of manufacture or maintenance of the drug or cosmetic to the concerned inspector.
Punishment for the Manufacture, Sale and Distribution of Drugs in Contravention to the Act
The penal provisions for the Manufacture, Sale and Distribution of Drugs in contravention to the Drug and Cosmetic Act are as follows:
- The manufacturer of the drug that falls under Section 17A or 17B and causes death or grievous hurt of a person will be punished with a fine not less than Rs 10 lakhs or 3 times the value of the drug confiscated.
Further, the manufacturer will also be liable to imprisonment for a term of ten years. It can also extend to life imprisonment.
The person convicted will also be liable to pay compensation to the victim or the person who had consumed such an adulterated drug. However, in the case of death, the manufacturer needs to pay compensation to the family of the deceased person.
- In case a drug falls under section 17A of the Act [other than the one mentioned in point (a)] and is being used without a valid license, then the manufacturer will be liable to pay a fine of Rs 1 lakhs or 3 times the value of the drug confiscated. He/she will also be liable for the imprisonment of a term of three years, which may extend to five years.
- The manufacturer of the drug that under section 17B of the Act [other than the one mentioned in point (a)] will be liable to pay a fine of Rs 3 lakhs or 3 times the value of the drug confiscated. He/she will also be liable for the imprisonment of a term of seven years, which may extend to life imprisonment.
- The manufacturer of a drug other than the one mentioned in point (a), (b), and (c), works in contravention of the provisions of the Act, will be liable to pay a fine not less than Rs 20,000. He/she will also be liable for an imprisonment of one year, which may extend to two years.
Punishment for the Manufacture, Sale and Distribution of Cosmetics in Contravention to the Act
- The manufacturer of a cosmetic that falls under section 17D or 17E of the Act will be punishable with a fine not less than Rs 50,000 or 3 times the value of the cosmetic confiscated. Further, he will be liable to imprisonment for up to three years.
- The manufacturer of a cosmetic other than the one mentioned above will be liable to imprisonment for up to one year along with a fine up to Rs 20,000.
Conclusion
After discussing thoroughly, it can be summarised that there are some restrictions and prohibitions imposed on the manufacture, sale and distribution of drugs and cosmetics, which is obligatory for every manufacturer to comply with.
Further, the Drugs and Cosmetics Act, 1940 is the governing law for every issue relating to drugs and cosmetics.
Lastly, reach out to Swarit Advisors to obtain Drug License in a straightforward and hassle-free way. Our experts rank high in providing on-time registration.
Read, Also: Drug License Rules and its Eligibility Criteria.