Types of Drug License in India

Gonica Verma | Updated: Aug 03, 2019 | Category: Drug
Drug License in India must be acquired by all those persons who are engaging in the business of the pharmaceutical industry. The classification of Drug license will depend on the categories of drugs and the business involved with them. Respective State Government will issue this License according to their eligibility terms.
In this article, we will discuss various types of Drug License in India and how to acquire this License.
Table of Contents
What is Drug License?
Drug License in India is a kind of permission by the concerned State Authority to the manufacturers, retailers, wholesalers, distributors and all those who are engaging in the business of pharmaceuticals for carrying out the business activities related to the pharmaceutical industry.
Respective State Drug License Authority issues this License under the Drugs and Cosmetics Act, 1940. There are three significant classifications of Drug License in India which may be further divided into further categories.
What are the different types of Drug license in India?
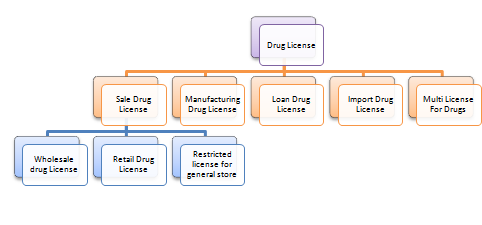
Manufacturing Drug License in License
This License must be acquired by the manufacturers of Allopathic, Ayurvedic, Cosmetics products or other drugs under the Drugs and Cosmetics Act, 1940. The respective State Government grants this License to the applicant.
Loan Drug License in India
Loan Drug License is issued to those authorities who do not have their own land for manufacturing their drugs but want to manufacture the products and services with their brand name on land of those are already issued with the License of manufacturing.
Import Drug License
This Drug License must be acquired by those persons who are using imported products for manufacturing drugs or those who are engaging in the distribution of imported drugs in India.
Multi License for Drugs
Multi-Drug License must be acquired by those entrepreneurs who are operating in more than one state. No one can sell drugs at more than one place without obtaining this Multi-Drug License.
Sale Drug License in India
The Sale Drug license in India is acquired by wholesalers and retailers who are engaging in the distribution of drugs in India. There are three different types of Drug License in India as follows:
Wholesale Drug License in India
The wholesale Drug License in India is issued by CDSCO (Central Drugs Standard Control Organization) to those wholesalers who are doing business in pharmaceuticals. The person who wants to obtain this License must have some degree or diploma in pharmacy from a recognized university and a minimum of one year experience in drug dealing. To get this License, the applicant must submit these following additional documents:
- Form-19
- A letter stating the purpose of this License
- Challan of fees
- Receipt of Property Tax
Retail Drug License
Retail Drug License in India is issued by Licensing Authorities to retailers who are doing business in pharmaceuticals, stand-alone pharmacists, etc. The applicant should be registered pharmacist under the State Pharmacy Council.
Restricted License for General Store in India
This License is issued under Forms 20A and 21A to dealers or persons, to sell drugs without the supervision of any qualified person.
What are the essential requirements for obtaining Drug License in India?
Area for the operations of the business
A minimum area of 10 sq meters is required to set up a medical shop or retail pharmacy. If they are operating with a wholesale one, and then the minimum of 15 square meters is mandatory.
Proper Storage Facility
There are several medicines and vaccines which need to be restored at cool places, so the requirement of A/C and refrigerators is necessary for the storage.
The requirement of technical staff
The presence of a qualified and experienced person is necessary to operate a retail pharmacy in India.
What are the documents required for obtaining the Drugs License in India?
- Passport size photograph of the owner(s)
- Identity proof of owners
- Address proof of owners
- Evidence for depositing the application fees
- Application form, Filled and signed
- Self-attested copy of the registered plant layout
- Utility bill as the certified office proof, in case of owned land
- Rent agreement, if applicable
- No-objection Certificate from Landlord
- Educational background, experience letter of the registered pharmacist
- Invoice of Refrigerator for business purpose
- Invoice of the purchase of Air-Conditioner
Additional documents required in case of partnership firm
- Partnership deed
- Complete list of partners
Other documents required in case of Private and Public Limited Company
- Certificate of Incorporation
- Memorandum of Association
- Article of Association
- Appointment of the concerned and authorized person who will be responsible for daily operations.
- Copy of the board resolution regarding the authorized person who will be responsible for the day to day operations.
Additional documents required in case of a pharmacist
- Copy for the diploma in pharmacy
- Registration Certificate from The Pharmacy Council
- Experience Letter, if any
- Affidavit of pharmacist
- Details of the educational certificate of a competent person
Browse through our articles on services provided at Swarit Advisors, and just let us know if we can help you with your company registration or tax filing or trademark registration.
What is the procedure for obtaining Drug License in India?
Step-1 Signing up on Website
Firstly, the applicants need to visit the website of their respective State Drug Licensing authority and register themselves by filling the registration purpose.
After that, the applicant has to fill his details, organization details, and contact details to generate OTP.
Step-2 Uploading documents
After filling up the form, the applicant has to upload documents; the documents checklist is already mentioned earlier depending on the formation of the Company.
Step-3 Payment of Fees
After uploading all the documents, requisite fees of the form should be submitting and generate challan of that for further processing.
Step-4 Securitization of Documents
After submitting the form, the licensing authority will scrutinize the form, and the DOC (District Coordination Officer) of the concerned district will inspect the organization and ensures that it comply with all the requirements.
After the verification and inspection, the DCO will forward the report to the SDCO of the zone for granting the License.
Step-5 Issuing Drug License
The report of SDCO will be forwarded to the respective State Drug License Authority, and, if the authority finds no discrepancy in the report, then it will issue Drug License to that person. Generally, it will take a minimum of 30 days for the issuance of a Drug license in India, if the applicant fulfilled all the requirements.
What is the validity of a Drug License in India?
Usually, the validity of the Drug License in India is five years.