Overview of CDSCO Cosmetic Import Registration
In the Indian scenario, the cosmetic industry is speedily growing, with heavy market demand for cosmetic and personal care products. Should manage the import of cosmetics products in India to ensure the quality, safety and performance of those imported into India need CDSCO Cosmetic Import Registration License from the ministry of health & Family welfare as an order under Rule 21 of Drugs Cosmetics Rule 1945. The certification is required from the primary regulatory authority for CDSCO Cosmetic Import Registration, obtained from the Central Drug Standard Control Organisation (CDSCO). With this regulation, can carry out import activities and sales of cosmetic items without any problems in India. The Drugs and Cosmetics Act, 1940 governs CDSCO Cosmetic Import Registration in India.
What is a Cosmetic in India?
In India, cosmetic means any material aimed to be sprayed, poured, scrubbed or introduced into the people's body, which is used for altering, cleaning, or beautifying the aspect, containing any article used as a part of cosmetic products.
Role of CDSCO for Cosmetic Import Registration in India
- Revision of the Cosmetic Rules, 2020 concerning the import and registration of cosmetic products;
- Inspection of various applications for No Objection Certificate (NGO) or explanation regarding the import of cosmetic products;
- Operate of NGOs or public or Consumer forums on issues related to the standard of cosmetic products;
- Replying to the Government harmony or Bureau of Indian standards (BIS) as and whenever needed;
- Examining CDSCO Cosmetic Import Registration applications into the nation as per the Drugs & Cosmetics Act requirements, 1940 and Rules.
- Operate public inquiries or PMOPGs or hearing regarding the CDSCO Cosmetic Import Registration procedure & providing guidance to it;
- Construction of draft replies to RTI, VIP references, Court cases, and Parliament questions related to cosmetics.
Who can request for CDSCO Cosmetic Import Registration
As per the Drugs & Cosmetic Act, 1945, CDSCO has listed the application which can be eligible to apply for CDSCO Cosmetic Import Registration in India:
- Authorised Agent: if the manufacturer has authorised an agent on their behalf to import cosmetics.
- A subsidiary of the manufacturer: in the case of any foreign cosmetic manufacturer, the subsidiary firm of India can apply on their behalf for the CDSCO Cosmetic Import Registration.
- Manufacturer: the cosmetic manufacturer can also apply for India's CDSCO Cosmetic Import Registration grant.
- Other Importer: if any Indian importer wants to import cosmetics in India from a foreign manufacturer can apply for this certificate or license.
Documents required for CDSCO Cosmetic Import Registration
Following are some important documents required at the time of CDSCO Cosmetic Import Registration:
- Covering letter: purpose (fresh or Endorsement of products or Pack Size or manufacturing site or Additional Sourcing Location or Re-Registration) must be mentioned along with guideline of earlier issued Import Registration Certificate and product category (whether registered or not).
- Authorisation from the manufacturer (duly authenticated)
- Filled in part-1 of the Second Schedule
- List of ingredients along with their percentage contents
- Specifications and testing methods: the specification of the document is required from the manufacturer's end. The protocol for testing Cosmetics & specifications is as per the standards specified in the Cosmetics Rules, 2020 for the applied product.
- Manufacturing licenses/undertaking for no provision of manufacturing registration: the primary certified copy of manufacturing Registration or Marketing Authorisation regarding registered products issued by the Regarding Authority from the country of origin or undertaking.
- Free sale Certificate (duly authenticated): this certificate is provided by the National Regulatory Authority of the Country for the appeal products or from the Indian Embassy of the country of beginning. List of products of free sale Certificate should be stamped & signed by providing authority. A Free Sale Certificate (FSC) should contain the declaration that the applied product is freely sold.
- Pack insert (If any)
- Non-Animal Testing Declaration
- Declaration for Heavy Metal and Hexachlorophene content.
- Other documents ( if any)
- Application in form COS-1
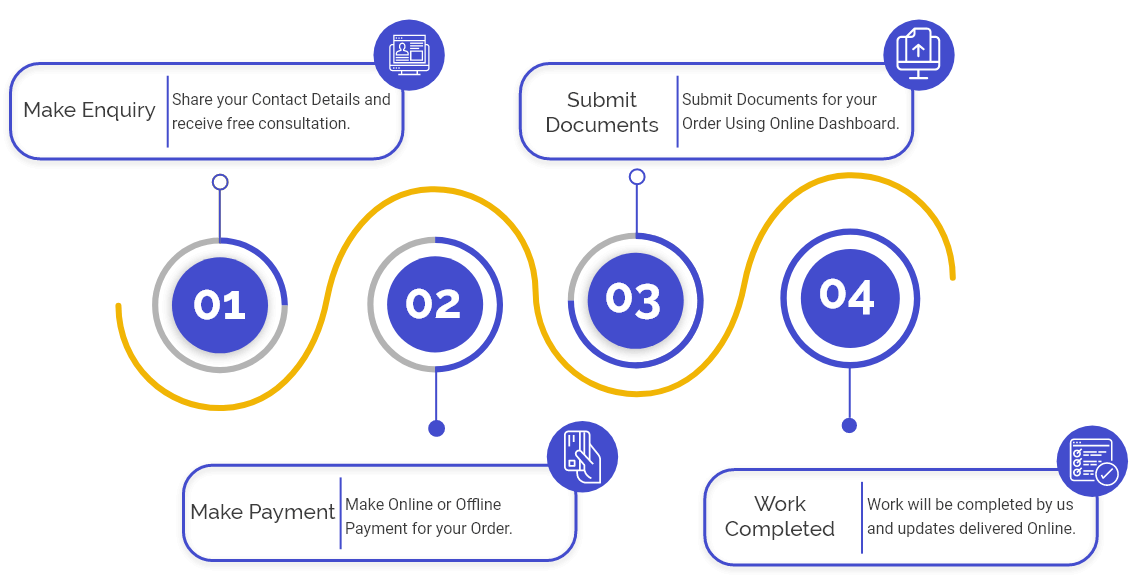
Process for CDSCO Cosmetic Import Registration in India
Following is the process the step for CDSCO Cosmetic Import Registration in India:
Step 1: Establish Cosmetics Classification: when the manufacturer resolves to register their products in India, the manufacturer must check the Gazette notice of CDSCO before finalising a device's regulatory status & classification.
Step 2: Nomination of an Indian Agent: The manufacturer should nominate an authorised Indian agent to link with the CDSCO. The Agent will accept a Power of Attorney to assist in cosmetic approvals and manage registration & importation in India.
Step 3: Filling the application form for CDSCO Cosmetic Import Registration: after the appointment of the Agent, the Importer or manufacturer of cosmetics shall need to submit the Registration form with all the documents and authorise fee on the CDSCO website to the DCGI (Drug Controller General of India) by simply logging on to the e-website of CDSCO and login into the e-website.
Step 4: allotment of CDSCO Cosmetic Import Registration Certificate: after the application form along with documents & authorisation fee is submitted on the online website of CDSCO, it may be sent to the DCGI Registration Authority a query via an inspection letter to the manufacturer or from the certified Agent of the Importer. After the authority is satisfied with the application & documentation, they may obtain a Cosmetic Import Registration.
Labelling of Proposed Products:
Following are the original label for the proposed cosmetic product or item and their variant (if any) according to Chapter VI of the Cosmetics Rules, 2020.
- Cosmetic products name;
- Manufacturer name and proper address of the manufacturer's place where the Cosmetic has been manufactured, or the country name where it has been manufactured as “Made in (Country Name)” should be there on the label;
- A unique Lot Number or Batch Number;
- must be maintained. Date of expiry and date of manufacture
- Manufacturing registration Number, the number id preceded by the letter M or M.L. No or M. L. No. shall carry on outer or inner labelling;
- A memorandum of the net contents stated in terms of weight for solids, fluid measure/ weight for semi-solids, fluid measure for liquids, integrated with numerical count if the content is sub-divided;
- In the case of cosmetics, where a danger exists; every inner label must indicate enough directors for safe use, caution or any particular direction required to be observed by the customer;
- In imported Cosmetics to be marketed in India, CDSCO Cosmetic Import Registration no. shall be cited in the unit pack label denoted by the letter R.C. or Reg. Cert. No. or R.C. No., along with the name & address of the Importer;
- If any cosmetic pack has only one label, the label must contain all the details essential to be present on both inner and outer labels under the prescribed rules;
- All the Instances, the ingredients list, present in a concentration of more than 1%, in any order and preceded by the words “INGREDIENTS”,
- The Cosmetic must comply with all labelling requirements specified in the relevant Indian standard as set by the Bureau of Indian Standard (BIS) for the cosmetics covered under the Ninth Schedule;
- Cosmetic should not be imported unless it is packed & labelled in accordance with these rules & label of imported cosmetics shall carry the Registration Certificate Number of the product & name and address of the owner of CDSCO Cosmetic Import Registration for marketing the concerned products in India.
How will SWARIT Advisors help you?
SWARIT Advisors assists you in applying CDSCO Cosmetic Import Registration Certificate. As mentioned above, the applicant may find it challenging to comply with documents and requirements. In this case, it is well to connect with a SWARIT Advisors, who will guide you on each procedure step.
Frequently Asked Questions
Cosmetic means any substance aimed to be scrubbed, poured, sprayed, or introduced within the human body, which is used for improving, purifying, or beautifying the appearance; this also contains any article used as a part of cosmetic products.
In India, the testing of cosmetics products on animals is banned.
The state government's specific licensing authorities issue the cosmetic manufacturing license.
You need to apply for re-registration six months before the certificate's expiry; it shall be considered the existing registration certificate to continue to remain in effect.
The primary purpose is to import cosmetics that should be regulated in India to ensure the quality & safety of cosmetics products being imported in India.