An Overview of InVitro Diagnostic device Manufacturing License
In India, the Drug & Cosmetic Act 1940 & Rules of 1945 regulate the manufacturing provision of InVitro diagnostic devices or kits or reagents (IVD). Before, there were no provisions for the registration of IVD. However, in the year 2017 Indian Government published an official notification for the registration of medical devices and IVD. In total, 462 products are classified as medical devices and 250 as IVD under the New Rules of the Government of India.
InVitro Diagnostic Devices are instruments, kits, reagents and systems that are deliberate to diagnose a disease or other health conditions, including regulate the state of health to cure, treat or prevent disease or its sequelae. Such products are intended for collecting, preparing, and examining specimens taken from the human body.
Classification of InVitro Diagnostic
According to the Medical Device Rules of 2017 classify, IVD Device as
Classification based on Risk
In Vitro Diagnostic, Medical Devices are categorized Based on Risk Parameters as Specified In Part II of the First Schedule as Under:-
- Low Risk – Class A;
- Low moderate-risk- Class B;
- Moderate high Risk- Class C;
- High Risk- Class D
Classification based on depressed use of Respective IVD
According to chapter II, Rule 4, Sub Rule (2) of medical device Rules 2017 and ground on Parameter advised in Part II of the First Schedule of Medical Device Rule 2017, the IVD are classified as by
- In-Vitro Device for HIV
- In-Vitro Device for HBV
- In- Vitro Device for HCV
- In-Vitro blood grouping sera
Regulatory bodies for InVitro Diagnostic Device Manufacturing License
State licensing Authority (SLC):
SLA is empowered to grant a license to manufacture Class A and Class B IVD in all states and UT in India. The manufacturing company need to submit their application to the state Drugs Control Authority having Jurisdiction over the manufacturing sites. The applicant must submit all relevant technical & administrative documents to the SLA requesting Licenses to manufacture IVDs.
Central Drugs Standard Control Organization (CDSCO):
CDSCO, by the Directorate General of health services and the Ministry of Health & Family Welfare, the Indian Government, is the central or National licensing authority for Class C and Class D IVD devices or kits in India.
Brief of Forms for application
|
Type of License
|
Risk / class |
Application Form
|
Approval Form
|
|
Manufacturing license |
A & B |
MD3 Application for grant of licensed manufacture for sale and distribution of Class A & B medical devices |
MD5 License to manufacturing for sale & distribution of Class A & B medical devices |
|
Loan license |
A & B |
MD4 Application for a grant to loan license to manufacture for sale and distribution of Class A & B medical devices |
MD6 Loan license to manufacture for distribution & sale of Class A & B medical equipment |
|
Manufacturing license
|
C & D |
MD7 Application for obtaining of license manufacture for sale and distribution of Class C & D medical devices |
MD9 License to manufacture for distribution & sale distribution of Class C & D medical devices |
|
Loan license
|
C & D |
MD8 Application for issues of loan license to manufacture for distribution & sale of C & D medical device |
MD9 Loan license to manufacture for distribution & sale of Class C & D medical devices. |
Document required to apply for InVitro Diagnostic Device Manufacturing License
- Covering Letter
- Application Form.
- Receipt of fees challan
- Documents of the Constitution of the firm, including
- Partnership deed in case of partnership firm
- MoA & Articles of Association for the company registered under the Companies Act, 2013
- Declaration of the total number of Proprietor/Directors/ partners/managing Director
- Documents of age and postal or residential address of all partners & directors
- Documents of Site ownership/rent agreement
- Additional documents to submit for the master file
- Declaration of Manufacturing Chemist.
- Declaration of Analytical Chemist.
- Documents of educational qualification, experience & approval certificates of proposed Manufacturing Chemist and Analytical Chemist; Appointment Letters; ID proof.
- Registration from District Industries Centre.
- Performance Evolution Report
- Copy of test license
- We are undertaking that the manufacturing site complies with the provisions of the Fifth Schedule. Additional documents are required if applying for a loan license on Form MD-4.
- Consent letter from a central manufacturing unit in case of loan license.
- Wholesale licenses of the applicant loan licensee.
- Valid manufacturing licenses & copies of product permission of the product in question of the principal manufacturer.
Procedures for obtaining InVitro Diagnostic Device Manufacturing License
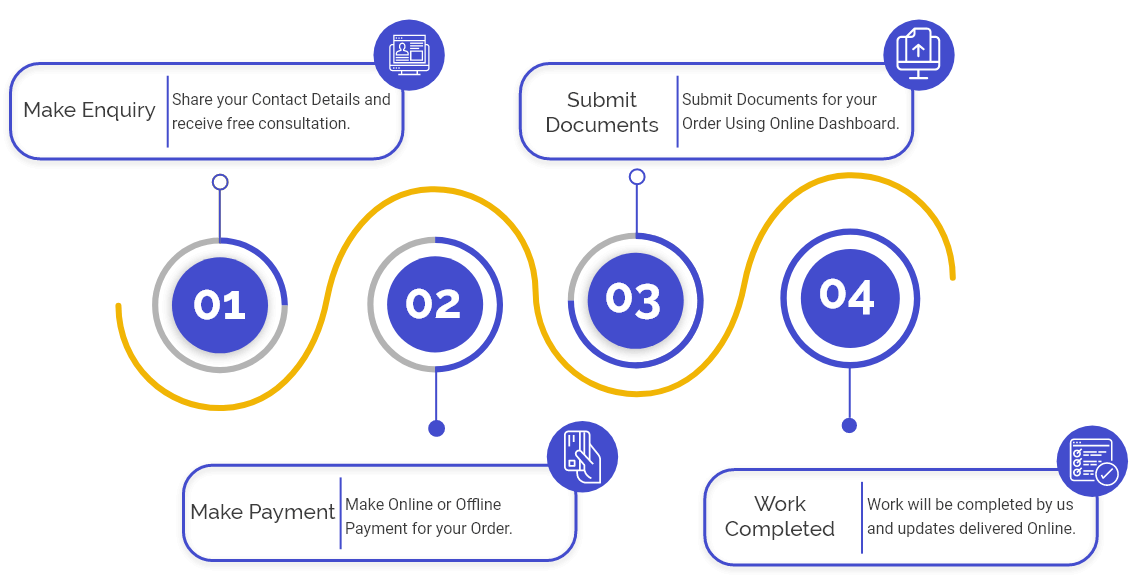
Step 1: Application to obtain of IVD Manufacturing License
The applicant must submit an application with the CDSCO in the requisite form, along with essential fees & challan specified.
Step 2: Scrutiny of Application
If any shortcomings/discrepancies are noted, the department raises the query & informs the applicant concerning the same. After scrutiny, if the application is in the actual order, it is prepared for an audit by the notified body according to the medical device rule.
Step 3: Audit of applicant premises by a notified body
If there is any Non-Compliance, the applicant need to improve it. The notified body then shares the audit and NC closure reports with the applicant.
Step 4: Inspection Of Audit Report
If the Audit report begins to be adequate after the examination, it is forwarded to the next step.
Step 5: Products Scrutiny.
The next step is scrutiny of the products' details; if the products comply with all norms & regulations, the application is considered for obtaining of license.
Step 6: Grant Of License
all the required norms & conditions are fulfilled, and the In Vitro Diagnostic Device Manufacturing License is granted to the applicant company.
How will SWARIT Advisors help you?
SWARIT Advisor is the most trustworthy platform to provide a wide range of services across India, including Government registration & licensing services. Swart Advisors on-time delivery of Government licensing & registration. Facilitate comprehensive support throughout the Registration procedure and quickly obtain InVitro Diagnostic Device Manufacturing License in India.
Frequently Asked Questions
Yes, all In -Vitro Diagnostic kits/reagents are regulated in India under the provisions. Of the Medical Device Rules, 2017.
Medical Devices & Diagnostics Division, Central Drugs Standard Control Organization (CDSCO), Directorate General of Health Services, Ministry of Health and Family Welfare, Indian Government.
The manufacturing company shall file their application to the State Drugs Control authority under whose Jurisdiction the manufacturing Premises is located. The firm shall submit all relevant technical and administrative documents to the SLA requesting a Licence to manufacture IVDs.
Shall warrant One Inspection with a gap of one year.
For each type of IVD, there is a requirement to maintain a ―IVD Master File‖. The Contents of the IVD Master File has been detailed in an appendix - II of the Fourth schedule.
The contents of the Plant Master File have been detailed in an appendix - I of the Fourth schedule.