An Overview of Medical Device Import license
In India, Medical device import is essential for every manufacturer. To import into India, the manufacturers have to fulfil quality and efficacy standards to enter the Indian market. In India, the Drugs & Cosmetics Act, 1940 and Rules, 1945 regulate the manufacturing, Sale, Distribution, and Import of drugs, Cosmetics, Medical Devices, and IVDs. In the importation of Medical Devices into India, it is mandatory to obtain Medical devices Import License by CDSCO; then, only you can import the medical devices into India.
The Central Government supervises regulatory authority over these articles imported into India through CDSCO, which is overlooked under the DCGI. The manufacture, sale, and distribution of the Medical Device Import License in India ensures the availability of potent, secure and quality medical devices based on scientific excellence and the best possible regulatory practices.
The Regulatory bodies are primarily responsible for Medical Device Import licenses in India.
The following are primary regulatory bodies that are answerable for the CDSCO Medical Device Import license in India:
- The Drug Controller General of India (DCGI);
- The Central Drug Standards Control Organisation (CDSCO);
- The import, sale, manufacturing, and distribution of different medical devices are regulated or managed under India’s Drugs & Cosmetic Acts and Rules.
Type of Medical Devices in India
- Notified Medical Device Importation: in India, for medical device registration, 37 device categories have been listed as ‘Notified Medical Devices’ under the CDSCO. These 37 devices have a separate procedure for registration. The manufacturer is mandatory to obtain the MD-14 by the CDSCO for these 37 devices, after which anyone can import into India. All these 37 devices have a separate procedure for import clearance, as explained below. However, only an individual/firm, or enterprise with a wholesale license and manufacturing license issued by the Drugs & Cosmetics Act, 1940 and Rules 1945, can do the import. Only they can be applicants for registration and import of notified medical into India.
- Non-Notified Medical Device Importation: the CDSCO conducted a new amendment on 11th February 2020, wherein apart from above mentioned 37 advise devices, all the other devices, which include – instruments, appliances, apparatus and implants, irrespective of being used alone or in combination for a various objective like prevention, treatment, analysis, allaying of any disease, investigation, alteration or replacement or support of the anatomy among others, will be managed by the legislation. The new amendment is called the Medical Device (Amendment) Rules, 2020 and will be voluntarily for eighteen months from 1st April 2020 to 1st October 2021. The import procedure is common to a notified medical device; however, the certificate of survey is not required. To import within India, the manufacturer must obtain a registration label to import the products to India.
The conditions to be satisfied the obtain a Medical Device Import License.
As per Rule 25A of the DCR, before the Medical Device Import License, the Licensing Authority will have regard to:
- Whether the property/units/sites where the imported substances will be stocked are enhanced with proper storage housing for preserving or keeping the properties of the medicine to which the import license applicant;
- The applicant ordinarily carries out information about the trade, occupation, or business.
Note: the licensing authority, on being satisfied that the Medical Device Import License requirements have been complied with, grants an import license in Form-10.
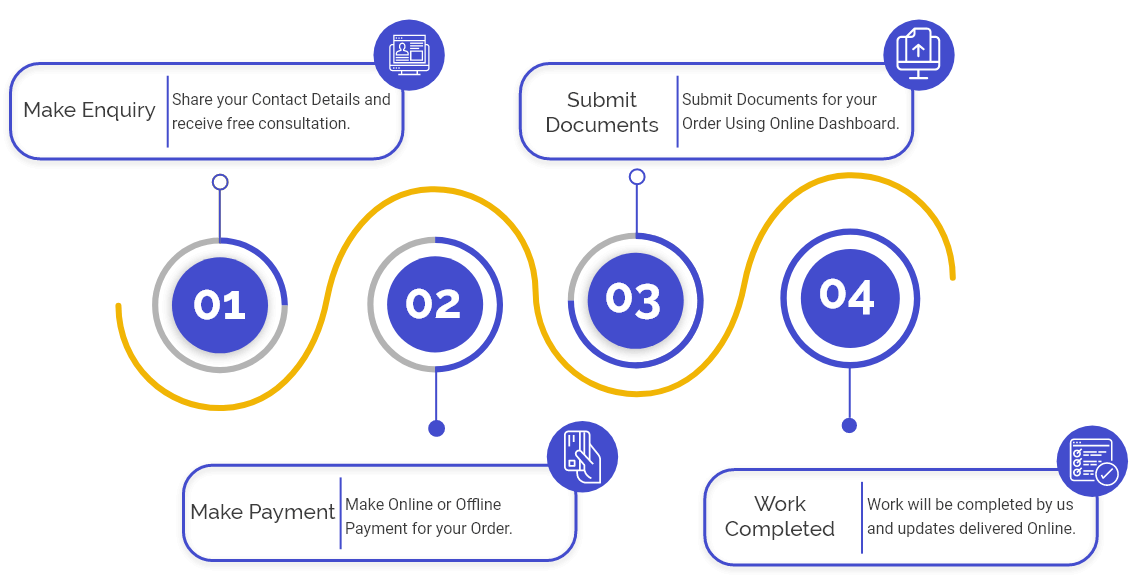
Procedure for CDSCO Medical Device Import License
Step 1: Application for the CDSCO Medical Device Import License in India concerning the premises and devices manufactured by the Manufacturer & meant for import into India are required to be made by the Importer or Manufacturer or their agent in India, in Form-40 and a manner as prescribed under Rule 24A of the Drugs & Cosmetics Rules. The application for the Medical Device Import License in India addressed to the Drugs Controller General of India shall be deposited at CDSCO.
Step 2: shall pay a fee along with the application as the CDSCO Medical Device Import License fee for the premises or units where the Manufacturer manufactures the devices aimed to be imported.
Step 3: Also, shall pay a fee for the Single Device Registration in India (which may comprise a variation in shapes or sizes without any changes in the method of use or material) and shall pay an extra fee for each additional device.
Step 4: The fee shall be paid via a challan as prescribed under the said rules;
Step 5: The information and undertakings required under Schedule DI and DII may be changed to suit the requirements of devices in place of everyday pharmacological products. The information shall include the following details:
- Details Of The Applicant:
- Company name, address, and contact number of the applicant;
- Address & name of Foreign Manufacturer (Manufacturing Premises);
- Copy of the Plant Master File;
- Name & address of the Importer;
- Name & address of the local authorised representative;
- Local Manufacturer, if any processing is being completed in the country.
- Product’s Information:
- Brand or Proprietary name;
- Device category;
- Method of use and intended use;
- Brief description of the device;
- Brief description of the manufacturing method & specification of the materials used;
- Variations in style, size, or shape of the device, if applicable;
- Recommended storage conditions;
- Warnings, contraindications, precautions for potential adverse events and alternative therapy, wherever applicable;
- Summary indications of any reported problems;
- Quantitative & qualitative particulars of the constituents;
- Details of standards to which the device conforms along with the copy of the standard;
- List of accessories & other devices or equipment to be used with the device. Other description information, comprising accessories packaged with the product;
- Packaging description comprising pack sizes;
- Labelling details conforming to Drugs & Cosmetics Rules, 1945;
- Promotional Literature & Physician manual in English;
- Medical speciality in which the device is used.
- Regulatory Status:
- Product approval from any other regulatory agency (separate evidence for the approval from each category):
- EU medical device directive (CE Certificate);
- Approval in any other country;
- US FDA Approval or Clearance;
- Japan or Australia, or Canada approval.
- List of nations where the device is being sold.
- Copy of EN or ISO Certification, if any, for the manufacturing facility.
- List countries where the device is withdrawn from sale with reasons if any.
- Master File (Information On Good Manufacturing Practices Employed By The Manufacturer To Make Sure The Device Quality):
- Shelf life of the device;
- Device Master File;
- Functionality Test protocol & report, if applicable;
- Stability data /statement of established stability of material used as applicable;
- Flow chart or manufacturing process;
- Risk Assessment as per ISO 14971;
- Quality Assurance procedures or Process Controls;
- Sterilisation process & Verification or Validation;
- Device GMP Certificate;
- Material or Component used;
- Final product testing/design inputs & outputs verification, if applicable;
- Biocompatibility & Toxicological data, wherever applicable.
- Devices Containing Medicinal Product:
- Clinical data & published articles, if any;
- For devices not authorised for marketing in the country, the applicant shall submit reports of clinical trials, sales details, a certificate of satisfactory use from the medical specialists about the use of the device and product complaints' details, if any;
- If the device incorporates a medical product, which is liable to act upon the body with action ancillary to that of the device, safety of the data, quality & usefulness of the medicinal substance used;
- Batch Release Certificate for products or items incorporating any medicinal substances of animal origin;
- If the device aimed to deliver medicinal products, data on compatibility with medicinal items or products.
(Medical devices with prior consent from any of the recognised regulatory authorities will be subjected to an abridged examination and only a summary of all the studies & details described above is to be submitted).
- Post-Market Surveillance:
- Handling of complaints;
- Procedures for distribution of records;
- Procedure for product recall;
- Adverse incident reporting.
- The undertaking of conformity concerning product standards, safety & effectiveness necessities and quality systems in the country of origin.
Step 6: The Registration Certificate shall be issued in Form-41 of the said rules;
Step 7: The Medical Device Import License application shall be made in Form 8 along with a fee in the form and manner prescribed under the Drugs & Cosmetics Rules.
How SWARIT Advisors Will Help You?
In India medical device import license is under CDSCO requires lots of conditions and documentation. New applicants may find it hard to fulfil such requirements & documentation. Hence, it is strongly recommended to contact SWARIT Advisors, who will guide you on each step of the licensing procedure. At SWARIT Advisors, we simplify the procedure for our respected clients by addressing all the legalities on their behalf.
Frequently Asked Questions
CDSCO, DCGI, Ministry of Health & Family Welfare, and Medical & Diagnostics Division in India.
However, if required, the applicant will be accountable for paying the fee for expenditure as may be required for the survey or visit to the site.
The medical device import license is valid for 3 years until the Registration Certificate is valid.
Yes, it is required.
You Should apply for a medical device import license. The application for re-registration provided the importer & India agent remains the same or a minimum of 3 months before the expiry of the Import License.
Yes, you apply for both registrations certificate and import licenses together.