An overview of Medical Device Manufacturing Registration
India is one of the top worldwide medical devices markets with a heavy contribution from device imports. In India, earlier, the manufacturers could sell medical devices without any jurisdiction. But since 2006, medical devices entering India should comply with the Indian Medical Device Regulations set by CDSCO. Presently, there is a proper system for medical device regulation in India for specific types of medical devices regulated under the Medical Device Rules.
The Central Drug Standard Control Organisation (CDSCO) manages medical devices in India. IVD (In-Vitro Diagnostics) is marketed in India. The Drug Controller General of India (DCGI) heard the CDSCO, and approval authority is shared between State Licensing Authority (SLA) and Canter Licensing Authority (CLA). In India, the Medical Device Manufacturing, import, sale & distribution of medical devices are controlled under Drugs and Cosmetics Act, 1940 and rules 1945. So, it is vital to obtain CDSCO Medical Device Manufacturing Registration in India.
The Regulatory Bodies are Responsible for CDSCO Medical Device Manufacturing Registration in India.
Following are the regulatory bodies that are responsible for CDSCO Medical Device Manufacturing Registration in India:
- DCGI (Drug Controller General of India) is then supervised for the manufacturing approval of certain drugs such as blood products, vaccines, large volume parenteral, rDNA derived, specified medical devices, and new drugs;
- The CDSCO (Central Drugs Standards Control Organisation) is the head regulatory body in India for pharmaceuticals & medical devices;
- In India, the manufacturing, import, sale, and distribution of medical devices are regulated under India’s Drugs & Cosmetic Acts and Rules.
Classification of Indian Medical Device
Medical devices under CDSCO of been classified into 2 categories, as discussed below-
Notified Medical Devices
- as per rule 4 of the Medical Devices Rules 2017, notified medical devices are those devices whose certification is mandatory.
- Notified medical devices include a total of 37 products which critical need to be CDSCO certified.
Non-notified Medical Devices
- According to the new amendment issued by CDSCO, a device such as instruments, apparatus, and implants, irrespective of their usage for the distinct objective, will fall under Non-notified medical devices.
- Non-notified medical devices include 313 products that critical need to be CDSCO certified.
Classification of Notified Medical Devices
individual classification systems exist for IVD and Medical Devices in India. Each classification is categorised into four different classes based on the extent of risk linked with these devices. Below is the table of four different categories of Indian Medical devices:
|
Device Class Risk |
|
|
A |
Low Risk |
|
B |
Low Moderate Risk |
|
C |
Moderate-High Risk |
|
D |
High Risk |
List of Medical Devices
|
S. No |
Name of the Device |
|
1 |
Disposable Hypodermic Syringes |
|
2 |
Disposable Hypodermic Needles |
|
3 |
Disposable Perfusion Sets |
|
4 |
Cardiac Stents |
|
5 |
In vitro Devices for HIV, HBsAg and HCV |
|
6 |
Drug-Eluting Stents |
|
7 |
Catheters |
|
8 |
Intra Ocular Lenses |
|
9 |
I.V. Cannulae |
|
10 |
Bone types of cement |
|
11 |
Heart Valves |
|
12 |
Scalp Vein Set |
|
13 |
Orthopaedic Implants |
|
14 |
Internal Prosthetic Replacements |
|
15 |
Ablation Devices |
Who can apply for CDSCO Medical Device Manufacturing Registration
Following is the list of who can apply for the CDSCO Medical Device Manufacturing Regulation in India:
- The Manufacturer has a listed office in India;
- The authorised Agent of the Manufacturer;
- The Subsidiary of the Manufacturer;
- Any other importer;
- Domestic Manufacturer
What are the apprise Bodies for Medical Devices in India?
- State License Authority (SLA):- SLA has been entrusted with the responsibility of providing manufacturing, loan, and wholesale license to medical devices under Class A and Class B. SLA assigns a notified body to validate the Quality Management System (QSM) requirements and technical review of medical devices under Class A and Class B manufacturers.
- Central Licensing Authority (CLA):- CLS IS entrusted to provide a license to all the imported medical devices in India, including manufacturing, loan, and wholesale license to medical devices under Class C and Class D. Moreover, CLA might provide services of a notified body to inspect the manufacturing site/ location and technical review of medical devices under Class C and Class D.
CDSCO Medical Device Manufacturing Registration- Manufacture of Medical Devices
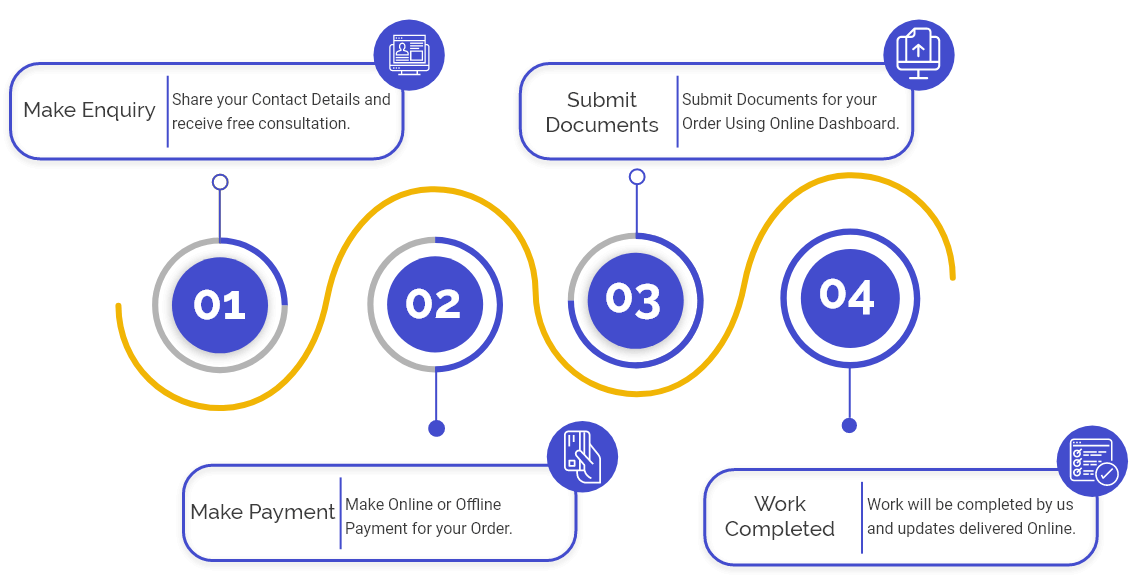
Step 1: Application for the certificate for the manufacture of these notified devices in India shall be made in Form-27 to the State Licensing Authority (SLA), accompanied by a fee in the form & format as prescribed in the proposed Rules along with a copy to the DCG Office.
Step 2: A 60 Days would be given for making the application for manufacture from the issuing date of these guidelines.
Step 3: in the case of devices being into the above-said categories, which have not been manufactured in the country before the informing date, no manufacture would be permitted subsequently without the competent authority's approval according to the norms given.
Step 4: the applicant shall submit the following details along with the application to licensing authority:
- Details of Manufacturing:
- Details like names, addresses of the manufacturing units and addresses of the company's directors and registered offices of the Manufacturer;
- A brief project notify showing the company's plan, a device to be manufactured, their viability and other important profiles;
- Copy of the plot Master File;
- Details of the standards obey by the company for product investigation & good manufacturing practices;
- Copies of ISO/any other acceptation, if required, obtained by the firm for its manufacturing area;
- A detailed clarification of the manufacturing procedure of the devices to be manufactured;
- modification, experience & name of technical staff under whose supervision will manufacture the devices.
- Details of the Product:
- Brand/Proprietary name;
- Device category;
- Method of use and intended use;
- Brief description of the device;
- Specification of the device;
- Variations in style, size, and shape of the device, if applicable;
- Recommended storage conditions;
- Warning, contraindications, precautions for potential adverse events and alternative therapy, wherever applicable;
- Summary indications of any reported applicable;
- quantifiable & comparative particulars of the constituents;
- List of attachments & other devices or equipment to be used in combination with the device. Description information, comprising attachment packaged with the product;
- Packaging explanation comprising pack sizes;
- Labelling details obey to Drugs & Cosmetics Rules, 1945;
- Promotional literature & Physician manual in English;
- Medical department in which the device is used;
- Testing resources are available in the manufacturing units for testing.
Step 5: To investigate medical devices that are new or do not have any benchmark certification, shell establishes the expert committees to examine in detail the instruction given by the applicant for the device evaluation.
Step 6: the committee, after finshing their assessment, forward the opinion regarding device suitability to the competent authority for permission to place the device on the market.
Step 7: After the joint verification and inspection, the SLA would forward the license to CLAA for approval.
Step 8: Shell granted the license in Form 28 of the said rules after due approval of CLAA.
HOW will SWARIT Advisors help you?
In India, medical device manufacturing registration under CDSCO requires many necessary documentation requirements. New applicants may find it challenging to comply with such requirements & documents. Hence, in such a case, it is advisable to contact SWARIT Advisors, who will guide you on each step of the licensing procedures. At SWARIT Advisors, we can simplify the process for our respected clients by addressing all the legalities on their behalf.
Frequently Asked Questions
THE CDSCO is India's primary legislative body for regulating and directing pharmaceuticals & medical devices.
Those such devices are for internal or external use in the treatment, diagnosis, mitigation, or determent of disease or confusion in human beings or animals, as may be determined from time to time by the central government of India through a notification in the Official Gazette after consultation with the board.
CDSCO, DGHS, Ministry of Health & Family Welfare, and Medical & Diagnostics Division.
It will be valid for three years from the date of its issuance.
In India, currently, only 40 – 50 medical devices require registration, and for all other devices that don’t require registration, the Manufacturer should get a No Objection Certificate (NOC) from the DCGI.
Class I present minimal harm to human, class II poses a higher degree of risk, and Class III applies to the high-risk types of medical devices.