Overview of CDSCO Cosmetic Manufacturing License
In India, presently, the cosmetic industry is expanding rapidly everywhere, from Television to social media sites. People use cosmetics from all over the world, whether they are men or women. As we know, that power comes with liability, so cosmetic manufacturing has attracted the Government of India due to its heavy requirement.
The Central Drugs Standard Control Organisation (CDSCO) control cosmetic products and the Drugs and Cosmetics Act, 1940 manages CDSCO’s Cosmetic Manufacturing License. After 2013, CDSCO became mandatory for all the imported cosmetics for sale to register with the Drug Control General of India (DCGI); before that, there was no such provision.
In today's Scenario, Users are excited to know the ingredients in products & made choices about which one is good to buy. Therefore, starting a new cosmetic will need to stick a unique label with its Registration Certificate Number.
Classes assess Cosmetic Products
- Deodorant soaps products;
- Hair Colorants products;
- Products for Hair setting;
- Product for lips;
- Skin-whitening products;
- Face masks products;
- Toilet soaps products;
- Emulsions, creams & lotions, oil, and gels for the skin products;
- Cosmetic powders;
- Face masks products;
- Antiperspirants and Deodorants;
- fixing hair, Products for waving, and straightening;
- Teeth & Mouth products ;
- Ant wrinkle Goods;
- Shower preparations such as foams, oils, salts, and gels;
- Hairdressing products such as lotions, lacquers, and brilliantine;
- Make-up and products removing make-up;
- Products for external intimate hygiene;
- Sunbathing products;
- Depilatories;
- Hygienic powders;
- After-bath powders;
- Tinted bases (pastes, liquids, powders);
- Shaving products (lotions, creams, foams);
- Toilet waters, perfumes and eau de cologne;
- Make-up powders.
Regulatory Authority for CDSCO Cosmetic Manufacturing Licence in India
The mainly regulatory Authority for CDSCO Cosmetic Manufacturing License in India is the central Drug Standard control organization (CDSCO). The CDSCO manages all cosmetic licenses and drugs. All the conditions related to the CDSCO Cosmetic Manufacturing License must be liaised and regulated by this body.
The law that regulates the cosmetic industry in India is the Drug & Cosmetic Act of 1940 And Rules, 1945. Apart from CDSCO, the Bureau of Indian Standards (BIS) brings out various standards for CDSCO Cosmetic Manufacturing License in India. These bodies also manage the ingredients in cosmetics.
Regulations for Cosmetic Manufacturing License in India
Under the Drugs & Cosmetic Rules, 1945, Schedule M-II grouping cosmetics into 11 product groups:
- Powders;
- Creams, lotions, milk, shampoos, cleansing, pomade, shaving creams, hair oils etc.
- Nail lacquers and Nail polishes;
- Alcoholic Fragrance Solution products;
- Hair Dyes;
- Lipsticks and lip-gloss;
- Depilatories;
- Preparations used for eyes;
- Aerosol;
- Toothpowder and toothpaste;
- Toilet soaps.
To manufacture any product onto the above-mentioned list, a license is required that has been attained from a licensing Authority which set up an inspection fee. Besides, the manufacturer needs to keep a check that the manufacture is done in the showing of skilled technical staff, and the lowest of one of the staff people should possess the following education requirement:
- Must be submitted under the Pharmacy Act, 1948.
- Must obtain a diploma in pharmacy by the Pharmacy Council of India under the pharmacy Act, 1948;
- Has passed the halfway exam with the chemistry as one of the subjects or any education the licensing Authority may consider fit.
Thus, before obtaining or refusing the license, the Licensing Authority must examine the overall premises where the operation is to be carried out. The inspector is to be allotted as per the Act. The inspection officer will submit the report to the Licensing Authority, which will decide whether to grant the license or not.
Eligibility Criteria for CDSCO Cosmetic Manufacturing Licence
Following are the parties eligible to get a cosmetic manufacturing license:
- A manufacturer company with a registered operating office in India.
- Any authorized agent of the said manufacturer; or
- A subsidiary company of the manufacturer.
Documents Required for CDSCO Cosmetic Manufacturing License
Following are some significant documents required for CDSCO Cosmetic Manufacturing Licenses:
- From-32 for manufacturing of cosmetics;
- Fee deposited challan;
- Blueprint of site plan and critical Plan;
- Partnership proof of the premises, in case of rent;
- Ground of possession of the premises;
- Recognized copy of proof of constitution of the firm;
- Declaration form;
- Affidavit of the unbelief of partners or directors or owned under Drugs and Cosmetics Act, 1940;
- Bio-data form;
- Affidavit letter of a registered pharmacist or skilled person regarding full-time working with the firm duly attested by a notary;
- Certified copy of enrolment Certificate or Experience Certificate of the Registered Pharmacists or skilled person and qualification certificates;
- Certification letter of the registered pharmacist or skilled person in charge, if an employed person.
Procedure for obtaining CDSCO Cosmetic Manufacturing License
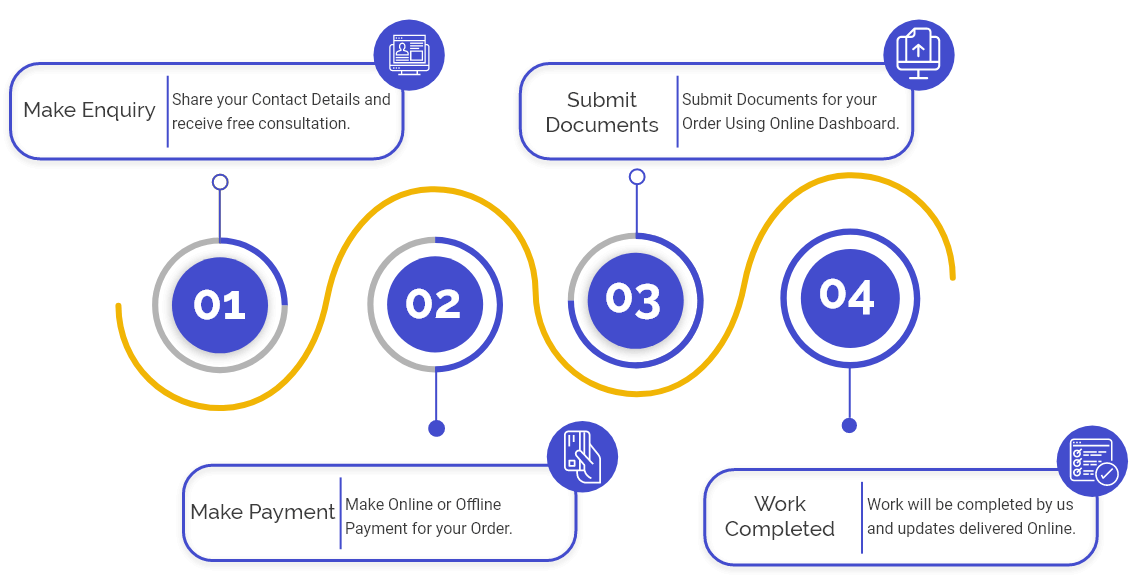
Following are some significant steps for obtaining CDSCO Cosmetic Manufacturing License:
Step 1: Fill out the application: primary, we need an application for a cosmetic Manufacturing License. We will help fill and drag the application on your behalf without any problem.
Step 2: Submission of documents: After filling out the application, you need to submit all the essential documents by mail, and we will generate your form under CDSCO and submit it.
Step 3: examination to be held: once we submit the application and documents to CDSCO, then they will examine the same.
Step 4: Issuance of License: Once the CDSCO department is satisfied with the documents and application, then they will grant the license, and we will mail you the license.
Penalty under the Drugs & Cosmetics Act, 1940
If the provision or rule under this Act regarding cosmetics is not complied with, imprisonment up to one year or maybe more than one year or a fine up to 1000/- rupees will be imposed. Both can be imposed on the first conviction and in the subsequent conviction, imprisonment can be extended up to two years and a fine up to 2000/-
Labelling Requirement for Manufacturers
Following are done major labelling requirements as laid down under the Drugs & Cosmetic Rules, 1945:
- The outer label should clearly show the components that are used in the manufacturing of the product;
- A individual batch number started with the letter B and must be mentioned the manufacturing batch with M on the label;
- should be mentioned the product name on both the inner or outer labels and in case the container is small & then the principal office and the pin code are fine;
- The inner label must hold the direction for use and a warning that may be essential.
Note: BIS Checks the product quality occasionally and amended the Indian Standard.
How will SWARIT Advisors help you?
SWARIT Advisors will help you fill and draft the application to obtain the Cosmetic Manufacturing Licences in India at less price. If you have any questions related to the CDSCO Cosmetic Manufacturing License, do not hesitate to contact us.
Frequently Asked Questions
This registration is required when an individual wants to manufacture a product that deals with skin cleansing or embellishment.
Within two months.
Yes, it is mandatory in India.
It is an executive body above the CDSCO, and this body executes all the functions regarding the compliance of cosmetics & drugs in India.
According to the Drugs & Cosmetic Act of 1940, a license on Form-32 is issued to manufacture, sell, or distribute cosmetics.