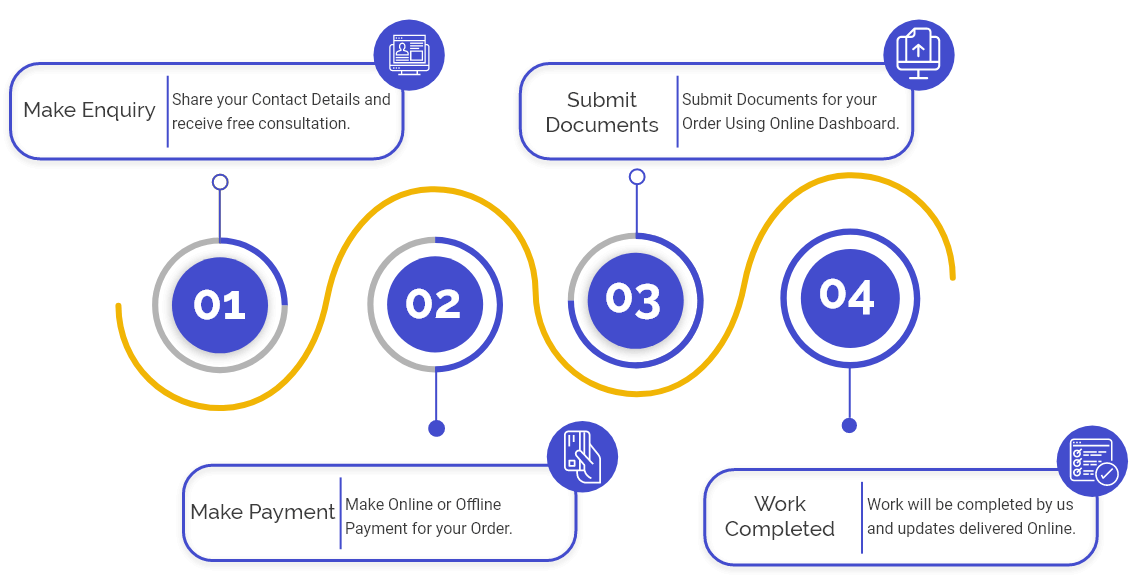
Overview of Dual Use NOC
Import of Drugs having Dual-use and drugs, which are used as Raw Material for the manufacturing of other drugs, requires permission from area offices of CDSCO.
Dual Use means drugs that can use in both pharmaceuticals and other industries. These are substances covered under the definition of drugs but are not used for medicinal objectives and are used in other industries such as the textile industry, chemical industry, food industries, etc. or are used as a starting ingredient or intermediate for the synthesis of other drugs.
In the case of imports of such drugs, an application for NOC, i.e. no objection certificate, needs to be procured from the area office of CDSCO.
What is CDSCO?
CDSCO Stand for Central Drugs Standard Control Organization, a governmental National Drugs Authority dedicated to the distribution and handling of the organisation on behalf of the Central Government that falls under the Drugs and Cosmetics Act, 1940 and Rules, 1940. This organisation has 6 Zonal Offices, four sub-zonal offices, thirteen Port Offices, and Seven Laboratories under CDSCO's management. The essential responsibility of CDSCO is control over the importation of various drugs, authorisation of new drugs, authorisation of new drugs Consultative Committee and DTAB, which stands for Drugs Technical Advisory Board and over various licencing authentication enacting as a Central License Approving Authority.
Why is Dual-Use NOC required?
There are several drugs accessible in the market that are not used in pharmaceuticals but instead used in manufacturing companies such as the chemical industry, textile industry, food and packaging industry, etc., used as an origin of raw material for the objective of fabrication of other drugs. Like drugs that are to be used for Dual Use and for the import of drugs, NOC, which support for No Objection Certificate, is compulsory.
The processes for the usage of drugs in mass quantity need Dual Use NOC usually. The Deputy Drug Controller shall issue NOC for the aim of Dual Use, which is not to be used for medical aims, India, for the importation upon submission of the application and compiling the needed legal undertakings. The importer is then needed to administer the granted NOC to Assistant Drugs Controller – 1 or the practical Officer present at the required port office to clear such products. The list of drugs introduced in Schedule D from Chapter-3 of the Drugs and Cosmetics Act, 1940 and Rules, 1945 is exempted by the conditions specified in the schedule. Even though the authorisation of NOC solely relies upon the Deputy drugs Controller, India, from any of the required Zonal Office.
Documents for application of Dual Use NOC
Following are documents that are needed for the Dual Use NOC Application procedure:
- Covering letter.
- Bill of either Invoice/Entry/Indent/Purchase Order/Sales Contract or Certificate of Analysis of all the batches imported and in case of High Seas sales: Copy of High Seas agreement (if any).
- Legal Undertaking on 100 rupees stamp paper as per proforma:
- Annexure-I, if the Actual User imports the drug
- Annexure-II, if the drug is registered for import with CDSCO, shall enclose details
- If the imported drugs are already registered with CDSCO, then shall also enclose information regarding the details.
- The d
- declaration is required regarding the drugs that will not use for pharmaceutical or any other medical objective.
- In the instance of use as an animal feed supplement or food supplement or conversion from one drug to another drug (quick manufacturing process or manufacturing flowchart)/Cosmetic use/ use in any other industry.
- Submit necessary permissions from the concerned departments and justifications of Dual Use.
- Detailed information on the previously permitted quantity of drugs.
What is Due Diligence?
The processes that require Dual Use NOC are needed to perform a Due Diligence before applying for the importation of required drugs.
The below-given points are necessary for successful due diligence:-
- The drugs to be imported must be approved via the country's laws, whether it's a single drug or a mixture of multiple drugs.
- The drugs need for importation have to be already registered.
- Check-up on the worldwide status of the drugs. For example, multi-vitamins are not acknowledged as drugs hence the laws applicable to their export and import are different.
- Technical Survey by Martindale Extra Pharmacopeia, etc.
Essential facts for filling out Dual Use NOC
Following is the necessary information regarding the Dual Use NOC procedure:-
- Before applying, the applicant needs to make sure that the application is complete with no errors.
- The clearance required for dual use is advisable to be made by the manufacturer or the agent authorised before the technical review stage for the consideration process before beginning the import procedure to avoid penalisation and preferably before two months of applying.
- The required documents, such as invoices, bills, etc., should clearly state the objective of the purpose of usage.
- Master formula records must be certified duly by Licensing Authority, which is compulsory for import applications.
- Importation of other drugs essential for purifying or sterilising imported drugs shall not be considered under Dual Use NOC.
- The permission for importing drugs by the genuine User is limited to one year.
- Suppose an application is made to the Port Official. In that case, it will be forwarded with a remark to the zonal head of CDSCO for analysis and consideration, preferably via email or fax. The NOC from the zonal head through email or fax will be sufficient for discharge.
- The Zonal Officer continue all required information in such cases.
How will SWARIT Advisors help you?
SWARIT Advisors has dedicated terms of compliance and regulatory professionals who can help provide end-to-end service related to Dual Use NOC. Our expert and experienced professionals ensure our client’s excellent services regardless of lengthy compliance procedures. We provide on-time delivery of government licensing and registration.
Frequently Asked Questions
Dual Use is drugs that can be utilised in pharmaceuticals and other industries like the chemical industry, textile industry, food and packaging industry, etc.
CDSCO stands for Central Drugs Standard Control Organisation, a governmental National Drug Authority dedicated to the distribution and handling of the company in place of the Central Government that pitch under the Drugs and Cosmetics Act, 1940 and Rules, 1945.
The processes that require Dual Use NOC are needed to perform a Due Diligence before applying to the importation of drugs required.
The permission period for Dual Use NOC by Actual User is one year.
The actual User is the importer, who imports essential chemicals for their product, and traders are importers who import the Dual Use drugs and sell them to other companies.