An Overview of InVitro Diagnostic Import License
In India, New Medical Device Rules, 2017, govern the import of InVitro Diagnostic. CDSCO (Central Drugs Standard Control Organization) has made an online platform, SUGAM, for submitting applications and issuing permits to Import IVD falling by any Category A, B, C, or D respective online form is needed to submit on SUGAM, which is a central Licensing Authority for such products.
Eligibility to apply for InVitro Diagnostic Device Import License.
The international manufacturers cannot straight apply as an applicant in India for license. They have to appoint an Indian Entity who is an authorised agent or license holder to submit their Application for registration in InVitro Diagnostic Device Import License. The authorised agent has either license to manufacture for sale or distribution or a wholesale license for distribution and sale that can apply to the CDSCO for a grant of Import License. An authorised agent is any firm or organisation that an overseas manufacturer has appointed to undertake InVitro Diagnostic Devices import in India through power of attorney.
Forms Required for Application and Permission
- MD 14: Application to get an import license for IVD Diagnostic Device.
- MD 15: Approval Form from central licensing agency to grant permission to import IVD Diagnostic kit.
- A, B, C, & D: Risk
- CDSCO: Authority to grant the license.
An essential document for InVitro Diagnostic Device import License
According to part II of the IV schedule of New Medical Rules 2017, the applicant is need to submit various documents for import registration:
- Covering letter:-
- The applicant has to mention whether the application is for registration or re-registration.
- The letter may also include a list of documents.
- An authorised signatory must duly sign all the documents.
- Details of Products to be imported
- Information on the manufacturing site.
- Regulatory and other Documents for attachment according to Form MD 14
- Power of Attorney
- ISO13485 Certificate
- TR 6 Challan
- GMP Certificate
- CE Design Certificate
- Declaration of Conformity
- PMS Report
- Business License or plant Registration Certificate
- Audit Report
- Constitution Details of the Indian Agent
- Good wholesale or manufacture license of the agent authorised in India for Registration.
- Quality certificate by overseas or manufacturer giving assurance of product quality.
- Must submit a free sale certificate (FSC) along with the application.
- Plan master files, including the below information
- Information on manufacturing procedure of InVitro Diagnostic Device
- Information on the source of antigen or antibody
- Characterisation of antigen or antibody
- Detailed composition of the InVitro Diagnostic Device
- Medical Flow chart procedure of InVitro Diagnostic Device
- Batch release Certificate
- Detailed Test Report
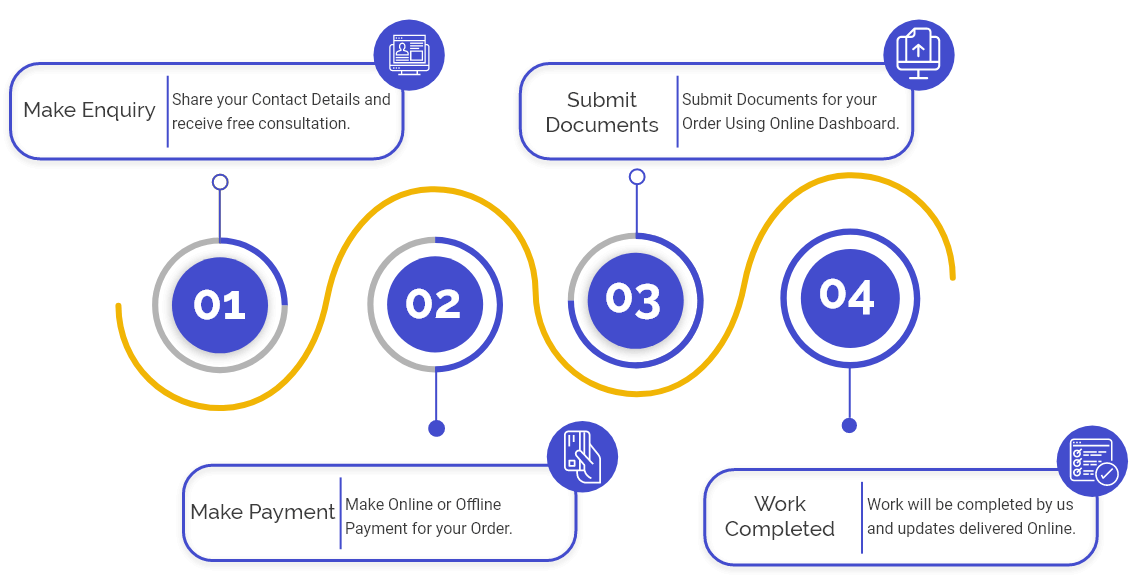
Procedure to apply for InVitro Diagnostic Import License
Step 1: Evaluation:-firstly evaluation of the product is to ascertain whether it fulfils the basic norms required to apply for an import license according to Medical Rules of 2017. The competent authority must do the process of evaluation.
Step 2: Classification:-if it requires according to Medical Device Rules, 2017, then the classification of competent authority does the InVitro Diagnostic Device.
Step 3: documentation:-Documents required according to the New Medical Device Rule 2017 must be kept ready and duly signed by the signatory authority.
Step 4: Appointment of an agent:-the agent who has a license to manufacture a Medical Device and in InVitro Devices for distribution & sale is appointed through Power of Attorney by an Overseas Manufacturer.
Step 5: Form Filing:-An application form is generated online. Must duly fill the form with all its attachments.
Step 6: Approvals by Agent:-The draft application form is to be approved & submitted by an authorised agent.
Step 7: Follow the up:-in case of any doubt, follow up with Regulatory Authority, and can do query management.
Step 8: Grant of Approval:-if all required conditions & guidelines are fulfilled, an InVitro Diagnostic Device import license is granted.
Validity of License
The InVitro Diagnostic Import License granted under Form MD 15 remains valid forever. The license reservation fees are paid as mentioned in the 2nd schedule before the expiry of 5 years from the date or its issue ends. This is done once and nicely if the license is not Suspended/cancelled under the Central Drugs Standard Control Organization.
Critical points for applying for InVitro Diagnostic Import License
must keep some essential points before applying for a Medical Device Import License.
- One must check the obtainability of "Free Sale Certificate: from GHTF countries.
- Check the number of manufacturing areas involved in the application.
- The Power of Attorney format must be the same manner as mentioned in the guidelines for Regulation of Medical Device rules of 2017.
- The documents needed for Device Master File and site Master File or any Technical Documents must also be according to the guidelines of Medical Device Rules, 2017.
- Apostillation and Notary must also be according to the Guideline of Medical Device Rules, 2017
- In India, an authorised agent for issuing of InVitro Diagnostic Device Import License. Devise only if they have a license to manufacture for distribution & sale or have a License of wholesale sale & distribution.
SWARIT Advisors support for Import License for InVitro Diagnostic Device
SWARIT Advisors has a dedicated expert of Regulatory and compliance professionals who can help you obtain an InVitro Diagnostic Import License. SWARIT Advisors have a team of skilled professionals to ensure that our clients get top-line services regardless of lengthy compliance processes. Swarit Advisors provide on-time delivery of government licensing & registration.
Frequently Asked Questions
Yes, In Vitro Diagnostic kits/reagents are regulated in India under the Drugs & Cosmetic Act 1940 & Rules 1945.
The diagnostic kits & reagents have been classified as "Notified" and "Non-Notified "
Following IVD kits/reagents are Notified as “Drugs” under Drugs and Cosmetic Act 1940.
- In-Vitro Diagnostic Devices for HIV
- In-Vitro Diagnostic Devices for HBV
- In-Vitro Diagnostic Devices for HCV
- In-Vitro Blood grouping sera.
If the application is absolute in all respects and information is in order, the licensing department may, within 3 months by the date of receipt of an application, issue an import license in From 10.
Same as IVDs meant for human beings.
Labelling should comply with the requirements of Rule 96 of the Drugs and Cosmetics Act and Rules